The mechanism of functioning of the mediator biosensor based on bacteria Gluconobacter oxydans immobilized in polyvinyl alcohol modified with ferrocenecarbaldehyde is considered.
Keywords: mediator biosensor, Gluconobacter oxydans, ferrocenecarbaldehyde
Amperometric biosensors are the most widespread and successful in terms of commercialization class of biomolecular electronics devices. The basis of microbial biosensors are immobilized cells of microorganisms. Significant progress in the creation of amperometric biosensors was made possible through the use of substances capable of transferring electrons from the active centers of enzymes to the electrode — mediators of electron transport [1]. In the catalytic cycle, the mediator interacts with the reduced form of the enzyme, and then diffuses to the surface of the working electrode, where a rapid electrochemical reaction with charge transfer occurs [2].
An important stage in the study of process parameters in the system “substrate — bacteria Gluconobacter oxydans — mediator — electrode” — is the choice of mediators that can most effectively interact with the enzymes of bacterial cells. The ideal mediator for converting the energy of cell metabolism into electricity should form a reversible redox couple on the electrode, and it should interact with enzymes, oxidizing substrates or reducing equivalents. For this, the standard potential of the mediator should be more positive than that of biological electron donors [3].
A voltage-current curve was obtained for a mediator biosensor, based on the bacteria Gluconobacter oxydans, immobilized into a gel of modified PVA, which is shown in Figure 1.
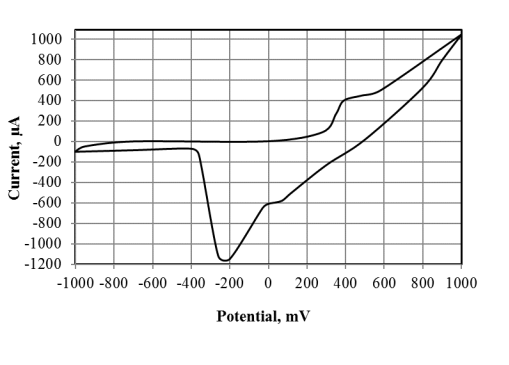
Fig. 1. Cyclic voltammogram of the formed biosensor (scan rate 50 mV/sec)
The cyclic voltammogram shown in Figure 1 demonstrates the thermodynamic reversibility of this system, as evidenced by two curves corresponding to the anodic and cathodic processes. The anodic peak corresponds to a potential of 500 mV, which is detrimental to microorganisms. The potential of the cathode peak corresponds to -200 mV, according to which the biosensor responses were recorded later.
The mechanism of operation can be represented by the following scheme (Fig. 2).
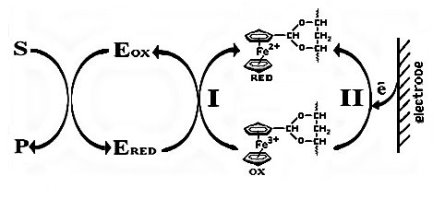
Fig. 2. Scheme of the functioning of the mediator biosensor
First, the substrate (S) interacts with the oxidized form of the enzyme (Eox) to form the oxidation product (P) and the reduced form of the enzyme (Ered). Next come two parallel reactions. Reaction I is the biochemical reduction of the oxidized form of the mediator by the reduced form of the enzyme. Reaction II — cathodic reduction of the mediator on the electrode.
The novelty of this scheme is that cathode reduction takes place at the electrode and, therefore, reactions involving a mediator take place in parallel. It is also convenient to use negative potential in biosensor analysis, because high values of potential negatively affect the receptor element of the sensor — living microorganisms.
References:
- Ponamoreva O. N. Biosensors and biofuel elements based on whole cells of microorganisms and enzymes isolated from them: review // Bulletin of TSU. Natural Sciences. 2009. Vol. 1. P. 138–157.
- Dzyadevich S. V. Amperometric biosensors. Basic principles of operation and features of sensors of different generations / S. V. Dzyadevich // Biopolymers and the cell. — 2002. — 18. — № 1. — P. 13–25.
- Alferov S. V. Physico-chemical aspects of charge transfer in the system «substrate — bacterial cells Gluconobacter Oxydans — mediator — electrode» in a biofuel element: dis.... Cand. chemical Sciences: 03.01.06 / Alferov Sergey Valerevich. — Moscow, 2010. — 127 p.

