The paper provides experimental confirmation of the heat of hydration of copper sulfate.
Keywords: heat of formation, crystalline hydrate, copper sulphate.
The heat of formation of crystalline hydrate CuSO4 · 5H2O will correspond to the reaction of addition of 5 moles of water to 1 mole of anhydrous salt according to the equation:
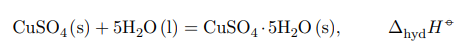
The thermal effect cannot be determined experimentally, because the formation of a new phase from liquid to solid phase is very slow and incomplete. Theoretical calculation of the thermal effect is carried out on the basis of Hess’s law on the basis of the following cycle:
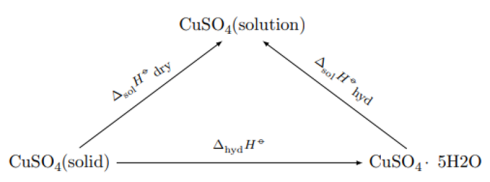
where ∆ sol H dry and ∆ sol H hyd — integral heats of dissolution of anhydrous salt and crystalline hydrate, respectively.
They are related by the following relationship:
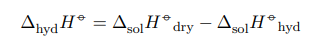
Also, the calorimeter constant must be determined before determining thermal effects.
For experimental determination of the calorimeter constant it is necessary to take a salt with a known heat of dissolution (for example: ammonium chloride). A beaker with 100 cm3 of distilled water and a magnetic stirrer is placed in the calorimeter. For uniform heat distribution and complete dissolution of the salt, intensive stirring is carried out for 3–5 minutes (preparatory phase). After the preparatory phase, 2 grams of anhydrous salt is placed in the beaker (Main Period). The end of the ”Main period” can be counted when the temperature begins to change linearly (at a constant rate). After another 10 counts are taken, this period will be considered the ”end period”.
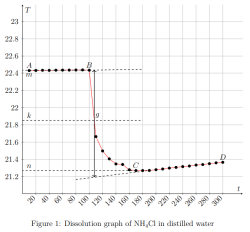
Determination of the calorimeter constant:
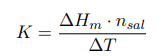
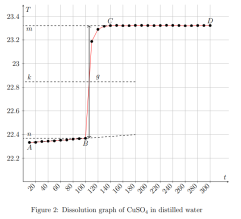
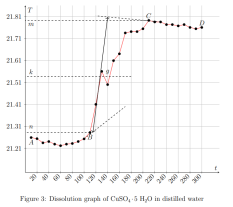
where ∆Hm — integral heat of dissolution of salt, expressed in J/mol; ∆T is the temperature change during experiment, measured in °C; n — quantity of substance, in moles; c is the specific heat capacity of the resulting solution (the heat capacity of dilute solutions of inorganic salts in water is practically the same and slightly differs from the heat capacity of water, specifically c = 4.184 J/(g ·K). Thus, by projecting points B and C onto the ordinate axis, then drawing a line parallel to OX, we obtain lines m and n, respectively. The line k is the midpoint of the segment mn, drawn to intersect with the graph, let us call the point of intersection — g. Extrapolating AB and CD, a vertical line passing through g, corresponding to ∆T, was found.
After plotting, the constant of the calorimeter is calculated:
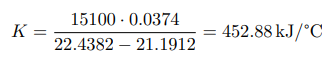
For experimental determination of the heat effect of dissolution of anhydrous salt CuSO4. A beaker in which 100 cm³ of distilled water is poured and a magnetic stirrer is placed is placed in the calorimeter. For uniform heat distribution and complete dissolution of the salt, intensive stirring is carried out for 3–5 minutes (preparatory phase). After the preparatory phase, 2 grams of anhydrous salt is placed in the beaker (Main Period). The end of the Main Period can be determined when the temperature begins to change linearly (at a constant rate). Another 10 counts should then be taken and this period will be considered the ”End Period”.
Determination of the integral heat of dissolution of an anhydrous salt:
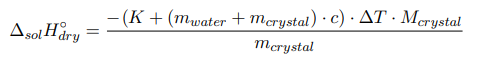
where m crystal , m water — the mass of anhydrous salt and water, respectively, g; M crystal — is the molecular mass of anhydrous salt, g/mol; c — the specific heat capacity of the formed solution; ∆T — the temperature change during the experiment, °C.
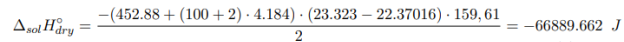
As in experiment 2 all conditions remain the same, only instead of anhydrous salt the crystalline hydrate is used.
Determination of the integral heat of dissolution of crystalline hydrate:
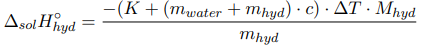
where m hyd , m water — the mass of crystalline hydrate and water, respectively, g; M hyd — is the molecular mass of crystalline hydrate, g/mol; c — the specific heat capacity of the formed solution; ∆T — the temperature change during the experiment, °C.
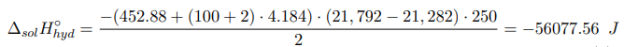
The heat of formation of crystalline hydrate is calculated from Hess’s Law by formula (2).
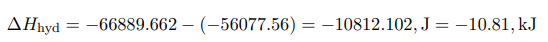
In this work, the heat of formation of copper sulfate was experimentally confirmed. The error in the calculation under standard conditions is about 3 percent.
References:
- S. V. Shtin: Physical Chemistry. Thermochemistry: manual for laboratory practice Chelyabinsk: Publishing Center SUSU, 2014.- 41 p.

