The article the most convenient method to obtain a novel 1,3-dioxane by Prins reaction. Prins reaction — a reaction producing a mixture of 1,3-glycol, and 1,3-dioxane by condensation of formaldehyde with olefins with acidic catalysts is a reaction that is an electrophilic compound of aldehydes and ketones which have double bonds. Therefore, according to the principle Prins reaction with a catalyst of sulfuric acid and acetic acid from the allyl alcohol and 1-hexene were obtained new derivatives of 1,3-dioxane. Synthesis conditions were reviewed and studied the physico-chemical properties of the resulting material and by IR spectroscopy determined the structure of matter.
In today's time, there are many ways of rational use of 1,3-dioxane and its derivatives. It is used as a stimulant for plant growth. 1,3-dioxane compounds as aromatic compounds found in the composition of the synthetic drug compound.
Keywords: derivatives of 1,3-dioxane, 1-hexene, chromatograpy, formaldehyde, IR-spectroscopy, allyl alcohol.
В статье рассмотрен самый удобный метод получения нового производного 1,3-диоксана с помощью реакций Принса. Реакция Принса — это реакция получения смеси 1,3-гликоля и 1,3-диоксана с помощью конденсации формальдегида с олефинами с кислотными катализаторами, то есть является реакцией электрофильного соединения альдегидов и кетонов, у которых есть двойные связи. Поэтому по принципу реакции Принс с помощью катализатора серной и уксусной кислоты из аллилового спирта и гексен-1 было получено новые производные 1,3-диоксана. Были рассмотрены условия синтеза, а также проведены исследования физика-химических свойств полученного вещества и с помощью ИК-фурье спектроскопии определили структуру вещества.
В наше время есть множество способов рационального употребления 1,3-диоксана и его производных. Он используется как стимулятор для роста растений. Соединения 1,3-диоксана, как и ароматные соединения, встречаются в составе синтетических лекарственных соединениях.
Ключевые слова: производные 1,3-диоксана, гексен-1, хроматография, формальдегид, ИК-фурье спектроскопия, аллиловый спирт.
In terms of chemical properties, 1,3-dioxane is a simple cyclic acetal. It will be in the «armchair» conformation. Soluble in water and mixed with all organic solvents. As the molecular weight increases in the homologous series, their solubility in water decreases. But cold water does not affect it much, because the rate of hydrolysis is proportional to the temperature of the water. Hot alkali solutions quickly break down this compound. The ring of 1,3-dioxane opens at room temperature under the action of liquefied acids according to aldehydes and glycols [2].
1,3-dioxane is found in the composition of the adhesive (in order to increase the elasticity of the adhesive), in the composition of the paint. And derivatives of 1,3-dioxane are found in the composition of semi-products and compounds in organic syntheses. Many of them are good solvents, derivatives of 1,3-dioxane are widely used in production, especially in the production of isoprene and other polymerizing materials [3].
Derivatives of 1,3-dioxane are also widely used as plasticizers. Plasticizers are substances that increase their elasticity and plasticity during processing and operation of polymer materials. Plasticizers facilitate the dispersion of the ingredient, reduce the temperature of technological processing of compositions, improve the resistance of the polymer to cold. EDOs plasticizer is an organic technical mixture of derivatives of 1,3-dioxanes, of which the main component is the symmetric form of 4-methyl-4 — hydroxyethyl-1,3-dioxane. For the production of linoleum, film coloring materials, vinyl, epoxy, polyester and other types of coatings are used [4].
Blick and his colleagues were interested in the formacological properties of 1,3-dioxane derivatives. Especially the properties of 2,2-dioxane 5-methyl-1,3-dioxane. As a result of the study, antispasmodic and antihistamine activity of 1,3-dioxane derivatives was established. Their application in the field of entomology has also been studied.
1,3-dioxanes are also widely used as a starting substance for the synthesis of substances such as varnish, Smola, determinant, antibiotic and insecticide. Syntheses based on Diene hydrocarbons are important in practice [5].
Some 1,3-dioxanes have valuable therapeutic properties. They are used as antidepressants and anticonvulsants for the treatment of neurodegenerative potalogies and diseases of the cardiovascular system. 1,3-dioxanes are compounds with anticancer, anti-inflammatory and antiviral properties [6]. In this regard, the main goal of our work was the synthesis of new derivatives of 1,3-dioxane based on the reaction of allyl alcohol and Hexene-1 with formaldehyde.
The synthesis of new derivatives of 1,3-dioxane was considered in the presence of sulfuric acid and acetic acid.
Condensation of allyl alcohol (1) and formaldehyde (2) was carried out in the presence of sulfuric acid in a 1:2 ratio at a temperature of 88–90º C under the condition of the Prince reaction. As a result, 5-hydroxymethyl-1,3-dioxane (3) was synthesized.
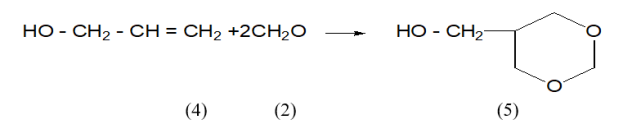
The results and physical constants for determining the structure of synthesized 5-hydroxymethyl-1,3-dioxane (3) are presented in Table 1.
The reaction of Hexene-1 (4) with formaldehyde (2) was also carried out in the presence of acetic acid in a 1:2 ratio at a temperature of 55–57º C under the condition of the Prince reaction, as a result of which 3-butyloxane-4-acetate (5) was synthesized (Table 1).
|
Compound |
profit, (%) |
Т. boiling, °С |
И K -спектр, (ν, см -1 ) |
R f (бензол: этил спирт, 7:3) |
|
5-гидроксиметил-1,3-диоксан |
67 % |
90 |
3350–3600 (О-Н); 2900–3000 (С-Н) . ; 1050 (С-О-С); 720–740 (CH 2 ) x . |
0,98 |
|
3-бутилоксан-4-ацетат |
55,4 % |
65 |
1740–1750 R-COO-; 2900–3000 С-Н . ; 1090–1200 –С-О-С-; |
0,83 |
Practical part
IR spectra were recorded in KVG tablets on the FSM 1201 IR-Fourier spectrometer, the course of the reaction was determined by the LH method on alufol plates (benzene: ethyl alcohol, 7:3) with iodine vapor.
Synthesis of 5-hydroxymethyl-1,3-dioxane in the presence of allyl alcohol
11.7 ml of allyl alcohol, 13.96 ml of formaldehyde and 0.3 ml of sulfuric acid were mixed in a Convex bottom flask with a mechanical mixer and a reverse cooler installed. At the boiling point of allyl alcohol, it was heated for 6 hours in an aqueous heater. The formed mixture was washed several times in a weak soda solution and benzene, and left to dry for a day with sodium sulfate on top. The next day, benzene was pumped out of the reaction mixture by a vacuum pump. As a result, 5-hydroxymethyl-1,3-dioxane is obtained (yield 67 %). Its boiling point is 88–90ºc, nd=1.4180.
Synthesis of 3-butyloxane-4-ol acetate in the presence of Hexene-1
29.5 ml of Hexene-1, 17.52 ml of formaldehyde and acetic acid were mixed in a Convex bottom flask with a mechanical mixer and a reverse coolant. At the boiling point of Hexene-1, it was heated for 6 hours in an aqueous heater. The formed mixture is washed several times in a weak soda solution and benzene, and left to dry for a day with sodium sulfate on top. The next day, benzene is pumped from the reaction mixture through a vacuum pump. As a result, 3-butyloxane-4-ol acetate is obtained (yield 55.4 %). Its boiling point is 65ºC, nd=1.4180.
Литература:
- Шепелевич И. С., Талипова Г.Р, Талипов Р. Ф. Успехи химии 1,3-диоксанов (статья) / в кн. «Новые направления в химии циклических ацеталей». ГИНТЛ «Реактив», 2009. С.36–50.
- И. Исагулянц, Т. Г. Хаимова, В. Р. Меликян С. В. Покровская. Синтез N-замещенных 4-аминометил-1,3-диоксанов / ХГС, 1995, № 1, с.21
- Й. Апьок, М. Барток, Р. А. Караханов, Н. И. Шуйкин. Получение замещенных ди- и тетрагидрофуранов по реакции Принса / 1996, Т.3, № 1–2, с.119
- В. И. Исагулянц, Μ.Г. Сафаров. Взаимодействие гептена-1 с формальдегидом в трифторусусной кислоте. / Нефтехимия, 1993, № 5, с.436
- Μ.И. Φаρбеρов, Η. К. Шемякина. Реакция Принса с участием аллилацетата, ЖОрХ, 1989, Т.25, № 3, с.488
- Ибатуллин У. Г., Файзрахманов И. С., Сафаров М. Г. Кетоны в сопряженной реакции Принса / ХГС, 1985, № 12, с.1688

