Allyl ester of natural oil acid obtained with reaction esterification of natural oil acids by allyl alcohol in presence of ion liquid N-methylpyrrolydone hydrosulphate have been investigated.. Yield of received allyl ester of natural oil acid is 85–90 %. The structure of allyl ester of natural oil acid has been determined by modern spectral analysis methods. Main parameters of allyl ester of natural oil acid used as modifier have been studied. Nitrile butadiene rubber and allyl ester of natural oil acid modified on the basis of phenol-formaldehyde oligomer adhesive composition is prepared in this research. Modification process of allyl ester of natural oil acids have been conducted by polycondensation method in the alkali medium on the unit equipped with thermometer, mixer and cooler. The amount of components has been given at (1, 15:1, 45:0, 25) ratio: phenol-1,15mol; formaldehyde -1,45 allyl ester of natural oil acids-0, 25. Modification process has been done in the alkali medium (pH=8–9). Reaction temperature is 95ºC, duration of the reaction 1,5 hour, and the yield of the product 70–75 %. Mainly, the physico-mechanical and physico-chemical properties of obtained oligomer have been studied. The new adhesive composition on the basis of nitrile butadiene rubber (SKN-26) and phenol-formaldehyde oligomer modified with allyl ester of natural oil acid is prepared and recommended in the use of adhesion of variety natural materials.
Keywords: phenol-formaldehyde oligomers, allyl ester, natural oil acid, modification, adhesive composition.
The adhesive composition is a difficult composition, besides, the oligomer consists of compactor, filler, solvent and etc. components. Physico-mechanical, technological and exploitation parameters of these compositions change depending on the content and the quantity of the content of its components. The adhesive ability of adhesive composition depends on the functionality, structure and the nature of macromolecule used as binder. Elastomer is added to reduce the fragility ability, to increase the adhesive ability of adhesive composition. Phenol-formaldehyde oligomer-based adhesives are superior to other adhesives because of their chemical stability, resistance to water, heat. These adhesives have a very high adhesive ability and moisture resistance in extreme weather conditions, at temperature and humidity [1].
In order to improve the exploitation properties and expand the areas of application of these adhesive components in the industry. the adhesives are modified with different organic compounds. The adhesive composition based on phenol-formaldehyde oligomer has a high resistance to mechanical effect, aggressive environment, high moisture content and high temperatures. Because it has a fixed three-dimensional structure. Modified phenol-formaldehyde oligomer is used as binder in numerous researches and also at the sticking of different materials, tree and wood constructions, glass, textile, ceramics material [2, 3].
Nitrile butadiene rubber and allyl ester of natural oil acid modified on the basis of phenol-formaldehyde oligomer adhesive composition is prepared in this research. For this purpose, the binder (oligomer) has been synthesized. Modification process of natural oil acids with allyl ester caused the change of many indicators of phenol-formaldehyde oligomer in positive direction and as a result of the exploitation, indicators of adhesive compositions have been increased [4–8].
Materials and Methods: Natural oil acid (NOA) was purchased from establishment «Karvan-L» (Azerbaijan), phenol was from of Moscow’s Component-«Reactant», formaldehyde was from Chemical Supply Company, Ltd., Kharkov. All these reactants were used without any further purification.
IR-spectrum of samples have been registered on IR-Furye microscope LUMOS (firm BRUKER Germany) within wave frequency 600–4000sm -1 .
Some physical-chemical indices of complex compounds, received on base of Baku natural oil acids have been determined. Crystallization temperature have been determined by standard — 5066-91, index of refraction
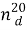
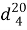
Synthesis of Allyl Ester of Natural Oil Acid. Esterification of the natural oil acid with allyl alcohol was conducted in three-neck flask, supplied by mechanical mixer, opposite cooler and Dine-Stark packing, catching the azeotrope mixture of benzene and water [9, 10]. At the reaction flask the NOA with allyl alcohol in correlation equal 1:1,2 were put. As solvent the benzene (100–150 ml) and as a catalyst, the ion liquid N-methylpyrolydone hydrosulphate in amount of 3 mol % were taken. Reaction was conducted with 80–90°C temperature, lasted up to 2,5–3 hours to achieve the balance state, and it is determined with the criteria of constant acid number. By that separation of reaction water is stopped. For esterification of unreacted NOA the received ester was washed by weak solution of 2 % potassium then by water up to neutral reaction. After distillation of the solvent, the vacuum distillation was conducted. Yield of received ester was up 85–90 %. Main parameters of allyl ester of natural oil acid used as modifier have been given in the table 1.
Table 1
Main parameters of allyl ester of natural oil acid
|
Name of parameters |
Amount |
Name of equipment |
|
Kinematic viscosity, mm 2 /sec. 40ºC |
2,75 |
Stabinger SVM ASTM D445 |
|
Density, kg/m 3 , 20ºC |
922,3 |
DMA 4500 M |
|
ASTM D 5002 | ||
|
Freezing temperature, ºC |
˂-60 |
Stanhope Seta |
|
ASTMD 2386 | ||
|
Emissivity |
1,4586 |
Abbemat 500 |
|
Iodine number J 2 /100 |
36,5 |
- |
|
Boiling point, ºC/6,65∙10– 4 МPа |
305–465 |
- |
Modification of Allyl Ether of Natural Oil Acid with Phenol-Formaldehyde Oligomer. Analysis of both the modified and unmodified phenol-formaldehyde oligomer, indicates that the functionality of the modifier causes increasing of main parameters. For comparison, the main parameters of unmodified phenol-formaldehyde oligomer have been researched [11, 12].
Modification process of allyl ester of natural oil acids have been conducted by polycondensation method in the alkali medium on the unit equipped with thermometer, mixer and cooler. The amount of components has been given at (1, 15:1, 45:0, 25) ratio: phenol-1,15mol; formaldehyde -1,45; allyl ester of natural oil acids-0, 25.
Modification process has been done in the alkali medium (pH=8–9). Reaction temperature is 95ºC, duration of the reaction 1,5 hour, and the yield of the product 70–75 %. Main the physico-mechanical and physico-chemical properties of obtained oligomer have been given at the Table 2.
Table 2
Main parameters of modified and unmodified phenol-formaldehyde oligomer
|
№ |
Parameter name |
Amount of parameters | |
|
FFO |
MFFO | ||
|
1 |
Yield, % |
65 |
70 |
|
Element contain, mass % | |||
|
2 |
carbon |
72,3 |
74,8 |
|
3 |
hydrogen |
7,5 |
9,4 |
|
4 |
Free phenol, mass, % |
9,7 |
3,6 |
|
5 |
Methylol groups, % |
11,2 |
8,9 |
|
6 |
Hydroxyl groups amount, % |
17,5 |
11,6 |
|
7 |
Density, kg/m 3 |
1250 |
1270 |
|
8 |
Molecule weight |
700 |
960 |
|
9 |
Melting temperature |
60 |
75 |
|
10 |
Adhesiveness, MPa |
2,2 |
2,8 |
|
11 |
Fluidity (Vik device) ºC |
105 |
156 |
Examples for Preparation of Adhesive Composition
- 100 mass part nitrile butadiene rubber (SKN-26), 50 mass part, phenol-formaldehyde oligomer modified with allyl ester of natural oil acids, 1.0 mass part sink-oxide, 3.0 magnesium-oxide 100 mass part ethyl acetate and 100 mass part aseton have been taken to prepare the adhesive composition. First nitrile butadiene rubber and phenol-formaldehyde oligomer modified with allyl ester of natural oil acid are solved separately in appropriate solvent. Then these solutions are mixed and gradually fillers add and during 1-hour mix at room temperature and adhesive composition obtain.
- 100 mass part nitrile butadiene rubber (SKN-26), 75 mass part, phenol-formaldehyde oligomer modified with allyl ester of natural oil acids, 2.0 mass part sink-oxide, 4.0 magnesium-oxide 150 mass part ethyl acetate and 150 mass part aseton have been taken to prepare the adhesive composition.
- 100 mass part nitrile butadiene rubber (SKN-26), 100 mass part, phenol-formaldehyde oligomer modified with allyl ester of natural oil acids, 3.0 mass part sink-oxide, 6.0 magnesium-oxide 250 mass part ethyl acetate and 250 mass part aseton have been taken to prepare the adhesive composition.
- 100 mass part butadiene-nitrile rubber (SKN-26), 125 mass part, phenol-formaldehyde oligomer modified with allyl ester of natural oil acids, 4.0 mass part sink-oxide, 10.0 magnesium-oxide 300 mass part ethyl acetate and 300 mass part aseton have been taken to prepare the adhesive composition.
- 100 mass part nitrile butadiene rubber (SKN-26), 150 mass part, phenol-formaldehyde oligomer modified with allyl ester of natural oil acids, 6.0 mass part sink-oxide, 13.0 magnesium-oxide 350 mass part ethyl acetate and 350 mass part aseton have been taken to prepare the adhesive composition.
- Adhesive composition is made by mixing of components.The content is shown in Table 3 .
Table 3
The content of adhesive composition
|
Contain |
Contain of invention samples | |||||
|
FFO |
MFFO | |||||
|
1 |
2 |
3 |
4 |
5 | ||
|
Nitrile butadiene rubber (SKN-26) |
100 |
100 |
100 |
100 |
100 |
100 |
|
Unmodified FFO |
50–150 |
- |
- |
- |
- |
- |
|
Phenol-formaldehyde oligomer modified with allyl ester of natural oil acids |
- |
50 |
75 |
100 |
125 |
150 |
|
Sink-oxide |
2–5 |
1 |
2 |
3 |
4 |
6 |
|
Magnesium-oxide |
4–12 |
3 |
4 |
6 |
10 |
13 |
|
Ethyle acetate |
150–350 |
100 |
150 |
250 |
300 |
350 |
|
Aseton |
150–350 |
100 |
150 |
250 |
300 |
350 |
The main indicators adhesive composition are studied and shown in table 4.
Table 4
The indicators of adhesive copmosition
|
Indicators |
Contain of invention samples |
Sample methods | |||||
|
FFO |
MFFO | ||||||
|
1 |
2 |
3 |
4 |
5 | |||
|
Appearance |
dark brown |
dark brown |
dark brown |
dark brown |
dark brown |
dark brown |
State standard, 10394–72 |
|
Viscosity (VZ-1 device) 20°C t, sec |
20 |
22 |
22 |
24 |
24 |
26 |
State standard 8420–74 |
|
Mass dry remain, % |
18 |
18 |
19 |
20 |
20 |
20 |
State standard 12172–74 |
|
The time of sediment |
28 |
28 |
30 |
30 |
32 |
32 |
- |
|
Breaking toughness, kN/m |
7.4 |
7.4 |
7.6 |
7.6 |
7.4 |
7.2 |
State standard 14760–69 |
|
Layout speed, m/sec. |
0.4 |
0.3 |
0.4 |
0.4 |
0.5 |
0.5 |
- |
The adhesive composition can be used as follows: The adhesive surface is cleaned with acetone or gasoline, then adhesive is applied and stored 2–3 minutes. It is not recommended to use a sticking product more than 15 hours. The adhesion consumption is 150–200 q/m 3 .
Results and Discussion. The esterification reaction of distillated natural oil acids with allyl alcohol in the participation of the catalyst N-methylpyrrolidone hydrosulfate proceeds by the following scheme:
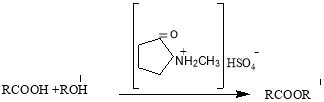
Where: R= –mixture of alkyl, isoalkyl and naphthenic radicals, R 1 — CH 2 CH=CH 2 -
The structure of allyl ester of natural oil acid has been determined by modern spectral analysis method on the Lumos IR Furye microscope (Bruker brand, Germany) at the 600–4000sm -1 absorbion range on Zn Se crystal.
Adsorption ranges of hydroxyl groups (OH) at 31.90–33.90 sm -1 , methylol groups (CH 2 OH) at 10.32–11.06 sm -1 , aromatic circle 12.61–1400sm -1 have been observed on IR spectral of modified and unmodified phenol-formaldehyde oligomer. Observation strips –C-H in CH 2 for 2924, 1455; for C=C 988,926 sm -1 , for C=C 3086, 1648sm -1 , for C=O 1736sm - 1, for C-O 1239 sm -1 , 1162 difficult esters for С-О bond.
It was not observed double bonds in modified oligomer of allyl ester of natural oil acid in IR analysis and modification is justified that bonds by breaking of these bonds. Modification of allyl ether of natural oil acid with phenol-formaldehyde oligomer is given like that.
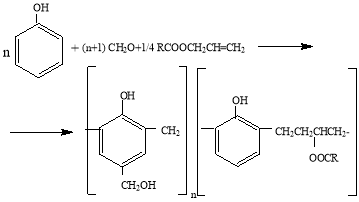
Here, R-C 13 -C 8 , m+n=3÷6 and n=2÷4
- Phenol-formaldehyde oligomer is modified with allyl ester of natural oil acid.
- Main parameters of modified oligomer has been studied.
- New adhesive composition is obtained on the basis of modified phenol-formaldehyde oligomer.
- Main indicators of the obtained new adhesive composition have been studied.
conclusion: Thus, as a result of the carried-out research, allyl ester of distillated natural oil acids obtained with the participation of the catalyst N-methylpyrrolidone hydrosulfate has a high yield and the shortest reaction time. Along with these, the ester obtained is not subjected to tarring, compared to other catalysts, which is of great scientific and practical interest. Allyl ester of natural oil acid is modified with phenol-formaldehyde oligomer and main parameters has been studied. The new adhesive composition on the basis of nitrile butadiene rubber (SKN-26) and phenol-formaldehyde oligomer modified with allyl ester of natural oil acid is prepared and recommended in the use of adhesion of variety natural materials.
References:
- Naibova T. M., Bilalova Y. M., Aliyev S. H. Adhesive composition. Patent AR № J2000 0035, 04.02.2000.
- Naibova T. M., Abdullayeva J. Q. Adhesive composition, Patent AR, J20030141.08.09.2003.
- Qarayev S. F., Bilalov Y. M., Naibova T. M. Adhesive composition, Patent AR, J2004 0147.22.11.2005.
- T. M. Naibova, N. M. Qaibova, A. A. Mamedova. Adhesive composition on the basis of modified oligomers, Collection of papers of the international scientific and technical conference “Modern achievements in the field of adhesives and hermetic materials, raw material and technology” Dzerjins, 17–18 September, 2013, p. 205–208.
- T. M. Naibova, N. A. Mamedova, S. A. Mammadxanova Adhesive composition, Pat. AR, J. 2018, 033.
- Shatirova M. J., Naibova T. M. Synthesis of glycidyl and thioglycidyl ethers of diacetylene series and their use as a modified of phenol-phormaldehyde oliqomers. Jzv. Vyssh Uchebn. Zaved Khim Tekhnol. 2019, v. 62, № 1, pp. 61–69.
- Petrova A.R, Zukina N. F., Dementeva L. A. Anikhovskaya L. I. Construction glue film, Journal of hermetically adhesive texnology, 2014, № 10.
- Shisulov O. F., Troshin D. P. Baulina I. S, Glukhikh V. V., Stoyanov O. V. Syntesis and properties of adhesives for wood-laminated plastics based on alcohol-soluble resol phenol cardanol formaldeide resins. Journal of hermetically adhesive technology, 2014, № 7.
- Mamedova N. A., Mamedkhanova S. A. Search of the optimal conditions and constructions of kinetic model of the reaction esterification of allyl alcohol with natural oil in presence of ion-liguid catalyst. International Research Journal of Engineering and Technology (IRJET), Volume 4, Issue 4, April 2017, Impact Factor value: 5., 181, p 1196–1204.
- Mamedkhanova S. A., Akhmedbekova S. F., Azizbeyli E. I. The study of the process of obtaining allyl ester of natural petroleum acids by IR spectroscopy. Oil refining and petrochemistry, No. 5, 2018, p. 22–25.
- Tarutina L. I., Pozdnyakova F. O. Spectral analyse of polymers. Chemistry 1986, 247 p.
- Kardawov D. A. The checking methods of sticks and adhesive compounds. M: Chemistry, 1976, 503 p.

