The study [1] was reported as the first one to collect larger samples of pancreatic tissue from recent onset type 1 diabetic patients. Laparascopic pancreatic biopsies had been performed earlier [2]. Here follows a comment on renal and pancreatic biopsy studies, performed in the course of the “pancreatic blood shunting into the systemic blood flow in insulin-dependent diabetics” [3] by the same researchers, who developed the concept of hypoplastic renal dysplasia [4–7]. This latter condition was described as follows: “Racemosely arranged glomeruli with single capillary loops, abundant rounded cells freely lying in the cavity of a capsule; single mesangial cells; irregular enlargement, loosening, and thinning of the basement membrane” [4], narrow extracapillary space, glomeruli having irregular form and singular capillary loops or total absence of capillaries [4, 5]. The descriptions could have been partly based on tangential sections of glomeruli (Fig. 1,3) or artifacts (Fig. 2a).
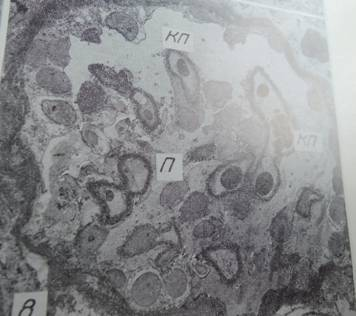
Fig. 1. Glomerulus with singular capillary loops (КП) and freely lying podocytes (П) x1100 [4].
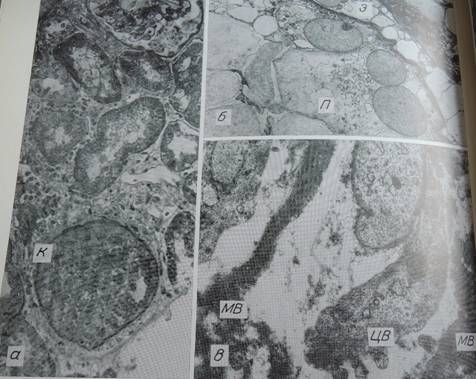
Fig. 2. Congenital nephropathy, case 1. a — absence of capillary loops in a glomerulus (K), compact arrangement of undifferentiated cells. Methylene blue-azure II-fuchsin stain. x400; б — podocytes (П) with large, pale, eccentrically located nuclei; large endothelial cells (Э) bulging into the capillary lumen x3200; в — fragments of disorderly arranged membrane-like material (MB), a cell with excrescences of cytoplasm (ЦВ) x20800
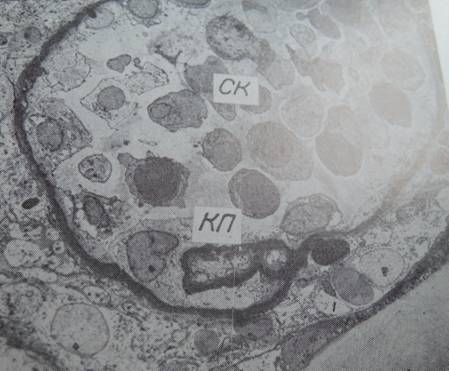
Fig. 3. Glomerulus with a singular capillary loop (КП), abundance of freely lying cells (CK), x1500
The authors of [4] were advised at that time that the concept of hypoplastic renal dysplasia should be verified on autopsy or nephrectomy material counting percentages of glomeruli “with single capillary loops” (Fig. 1,3) [4]; but it has not been done, and the concept has been persisting. For example, hypoplastic dysplasia was diagnosed as a principal renal condition in 8 from 34 patients aged 9–54 years with nephrotic syndrome and histologically minimal glomerular changes [6]. At the same time, Alport syndrome was not mentioned among 4440 cases, diagnosed by renal biopsy at the same institution, overviewed in [8]. The concept of hypoplastic dysplasia as a special form of nephropathy [4], discussed with clinicians performing biopsies, could have interfered with the diagnosis of Alport syndrome, having certain ultrastructural features in common with hypoplastic dysplasia as per [4–6]. Differential diagnosis of hypoplastic dysplasia with Alport syndrome was not mentioned in [4–6]. Note that Alport syndrome has significant genetic implications. Moreover, indications for renal and pancreatic biopsies applied by the same authors in research [9,10] should be questioned, considering the questionable morphological descriptions partly cited above. Today, the same authors apply the term hypoplastic dysplasia to the glomerular changes in congenital hydronephrosis and other congenital renal conditions (where intra-operative excision renal biopsies have been collected), interpreting them as “inborn nephropathy” reportedly affecting a majority of glomeruli [11–13]. It should be commented that coincidence of two conditions of different nature: inborn glomerulopathy and hydronephrosis with secondary pressure atrophy of renal parenchyma appears to be improbable. Other analogous studies were discussed in [14, 15].
The same researchers collected 60 pancreatic excision biopsies 5x5 mm in size [16] in the course of “pancreatic blood shunting into the systemic blood flow in insulin-dependent diabetics.” [3] From 1986 through 1994, 409 of such procedures were performed in type 1 diabetic patients by this research group [3]. From the same patients, 51 renal core biopsies were collected [16]. Apart from [17–25], we have found in the literature no analogues of this treatment modality of type 1 diabetes. The method was applied also in type 2 diabetes with severe hypertension [25]. The physiological mechanism, explaining for reported effectiveness of the shunting in diabetics, was delineated as follows: “The operation allows shunting of the venous blood flowing from the pancreas into the systemic blood flow, which should reduce the effect of glucagon on the liver, improve the correlation between injected insulin and endogenous glucagon both in the liver and in peripheral tissues.” [3] The anti-diabetic effect of the above-named surgery was generally moderate both in humans [3,19] and in preceding experiments [26]; whereas thrombosis-related hazards [18,20], postoperative acidosis [21–23], peritoneal adhesions and other complications [24] were pointed out. Acidosis was designated as a characteristic phenomenon [21], which agrees with the known fact that surgical stress can cause hyperglycemia and ketosis in diabetics [27].
Morphological descriptions of pancreatic and renal biopsies in type 1 diabetes mellitus included the following: islets of Langerhans “containing B-cells with destructive changes” [9], presence of endocrine-like cells in the acini and among the cells of the inter-acinar ducts [28,29], glomerulonephritis and mesangiolysis as consecutive stages of diabetic glomerulosclerosis [10], frequent mesangial interposition with displacement of mesangial cells to the peripheral capillary loops and formation of double-contour glomerular basement membranes [10,30], which is partly at variance with usual morphological descriptions [31–35]. In particular, glomerulonephritis, if detected in diabetic patients, has been interpreted as a superimposed condition or a complication [34,35]. Collection of renal biopsies from diabetic patients for research was planned in advance [36].
In conclusion, the purpose of this paper was to remind that, performing renal or pancreatic biopsy, the risk-to-benefit ratio should be kept as low as possible [14]. In particular, pancreatic biopsy is associated with risks [1,32,37]. Quality of morphological examination should be taken into account in determining indications to renal and pancreatic biopsies in each particular case. Finally, in the author’s opinion based on the literature overview, indications to the pancreatic blood shunting into the systemic blood flow in diabetics have not been sufficiently elaborated, which can pertain also to angiographic procedures involving catheterization of renal and splenic veins as well as arteriography described in [3].
References:
1. Krogvold L, Edwin B, Buanes T, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia. 2014, V 57, p. 841–3.
2. Imagawa A, Hanafusa T, Tamura S, et al. Pancreatic biopsy as a procedure for detecting in situ autoimmune phenomena in type 1 diabetes: close correlation between serological markers and histological evidence of cellular autoimmunity. Diabetes. 2001, V 50, p. 1269–73.
3. Galperin EI1, Diuzheva TG, Petrovsky PF, et al. Results of pancreatic blood shunting into the systemic blood flow in insulin-dependent diabetics. HPB Surg. 1996, V 9, p. 191–7.
4. Severgina ES, Pal'tsev MA. Hypoplastic dysplasia as one of the forms of nephropathy. Arkh Patol. 1989, V 51, N 10, p. 58–63.
5. Varshavskii VA1, Proskurneva EP, Gasanov AB, et al. Subdivision of certain morphological variants of chronic glomerulonephritis. Arkh Patol. 1999, V 61, N 5, p. 40–6.
6. Severgina ES. Ultrastructural heterogeneity of «minimal changes» in the kidney glomeruli, detected by light optics. Arkh Patol. 1991, V 53, N 2, p. 53–8.
7. Jargin SV. The concept of hypoplastic renal dysplasia can interfere with the diagnosis of Alport syndrome. CMAJ, February 14, 2014; http://www.cmaj.ca/content/102/4/396.citation/reply#cmaj_el_716893
8. Dzhanaliev BR, Varshavskii VA, Laurinavichus AA. Primary glomerulopathies: incidence, dynamics and clinical manifestations of morphological variants. Arkh Patol. 2002, V 64, N 2, p. 32–5.
9. Severgina ES, Diuzheva TG. Morphologic and functional changers in B-cells and vessels of the islands of Langerhans in patients with insulin-dependent diabetes mellitus. Arkh Patol. 1996, V 58, N 5, p. 40–7.
10. Severgina ES, Ponomarev AB, Diuzheva TG, et al. Diabetic glomerulonephritis — the first stage of diabetic glomerulopathy. Arkh Patol. 1994, V 56, N 4, p. 44–50.
11. Severgina LO, Leonova LV, Severgina ES, et al. Coupling between the hemodynamic parameters and the morphological changes in the kidney in children with congenital hydronephrosis. Arkh Patol. 2011, V 73, N 2, p. 14–7.
12. Leonova LV, Severgina ES, Popova OP, et al. Transforming growth factor as a marker beta of nephrogenetic disturbance in congenital obstructive uropathies. Arkh Patol. 2007, V 69, N 4, p. 35–8.
13. Cheskis AL, Severgina ES, Leonova LV, Ostapko MS. Status and development of the kidney after surgical treatment of hydronephrosis in children. Urologiia. 2002, N 4, p. 39–43.
14. Jargin SV. Renal biopsy research with implications for therapy of glomerulonephritis. Curr Drug ther. 2012, V 7, p. 263–7.
15. Jargin SV. Chernobyl-related bladder lesions: new interpretation required. J Interdiscipl Histopathol. 2014; doi:10.5455/jihp.20140127124925
16. Severgina ES. Morphology and pathogenesis of insulin-dependent diabetes mellitus. Habilitation thesis. I. M. Sechenov Medical Academy, Moscow, 1995. (Russian)
17. Siplivyi VA, Beresnev AV. The late results of deportalization of the pancreatic blood flow in patients with type-1 diabetes mellitus. Klin Khir. 1998, N 11, p. 9–12.
18. Nikonenko AS, Kovalev AA, Zavgorodnii SN, Volkova NA. Surgical treatment of insulin-dependent diabetes mellitus and its complications. Khirurgiia. 1996, N 2, p. 81–3.
19. Shraer TI, Rozina NS. Late results of pancreatic blood outflow deportalization and its significance in the combined modality treatment of diabetes mellitus. Probl Endokrinol. 1992, V 38, N 5, p. 49–52.
20. Torgunakov SA, Torgunakov AP. Possible causes of thrombus-related hazard of a distal splenorenal venous anastomosis. Angiol Sosud Khir. 2010, V 16, N 4, p. 184–8.
21. Torgunakov AP. Renoportal venous anasomosis. Kemerovo Medical Institute, 1992. (Russian)
22. Ivanov PA, Golikov PP, Shcherbiuk AN, et al. Characteristics of the postoperative period in diabetes mellitus type 1 in patients with distal splenorenal anastomosis. Sov Med. 1990, N 2, p. 17–9.
23. Gal'perin EI, Shraer TI, Diuzheva TG, et al. Experimental basis and initial clinical experience with the surgical treatment of diabetes mellitus. Khirurgiia. 1987, N 2, p. 64–70.
24. Gal'perin EI, Diuzheva TG, Kuzovlev NF, et al. Surgical correction of metabolism in diabetes mellitus. Khirurgiia. 1988, N 9, p. 6–11.
25. Putintsev AM, Shraer TI, Sergeev VN, et al. Variants of surgical management for severe arterial hypertension combined with type 2 diabetes mellitus. Angiol Sosud Khir. 2010, V 16, N 2, p. 120–5.
26. Gal'perin EI, Kuzovlev NF, Diuzheva TG, Aleksandrovskaia TN. Approaches to surgical treatment of diabetes mellitus (experimental study). Khirurgiia. 1983, N 1, p. 13–20.
27. Williams G, Pickup JC. Handbook of diabetes. 2nd edition. Blackwell Science, Oxford, 1999.
28. Severgina ES, Diuzheva TG, Razgulina LE, Stakheev IB. Is localization of B-cells in the acini a normal condition or the sign of compensatory process in insulin-dependent diabetes mellitus? Arkh Patol. 1992, V 54, N 12, p. 18–23.
29. Severgina E, Dyuzheva T, Paltsev M. Acinar B-cells in pancreas in insulin-dependent diabetic patients. The right to exist. Pathol Res Pract. 1993, V 189, N 3, p. 298–9.
30. Severgina ES, Ponomarev AB, Diuzheva TG, et al. Diabetic glomerulosclerosis — a prolonged stage of diabetic glomerulopathy. Arkh Patol. 1994, V 56, N 4, p. 50–5.
31. Rosai J. Rosai and Ackerman's surgical pathology. Mosby, Edinburgh, 2004.
32. Spencer J, Peakman M. Post-mortem analysis of islet pathology in type 1 diabetes illuminates the life and death of the beta cell. Clin Exp Immunol. 2009, V 155, p. 125–7.
33. Richardson SJ, Morgan NG, Foulis AK Pancreatic Pathology in Type 1 Diabetes Mellitus. Endocr Pathol. 2014; doi: 10.1007/s12022–014–9297–8
34. Dizdar O, Kahraman S, Gençtoy G, et al. Membranoproliferative glomerulonephritis associated with type 1 diabetes mellitus and Hashimoto's thyroiditis. Nephrol Dial Transplant. 2004, V 19, p. 988–9.
35. Hironaka K1, Makino H, Ikeda S, et al. Nondiabetic renal disease complicating diabetic nephropathy. J Diabet Complications. 1991, V 5, p. 148–9.
36. Severgina ES, Ponomarev AB. Patho- and morphogenesis of diabetes mellitus and early diabetic nephropathy. Arkh Patol. 1988, V 50, N 4, p. 80–5.
37. Atkinson MA. Pancreatic biopsies in type 1 diabetes: revisiting the myth of Pandora's box. Diabetologia. 2014, V 57, p. 656–9.

