The aim of this research was to make quantitative and comparative assessment of the main structural proteins’ expression which compose the contractile apparatus and the cytoskeleton of the contractile cardiomyocytes of mature females and newborn rats exposed to chronic hemic hypoxia. The research was carried out on mature female Wistar rats (6–6,5 months old, body weight 200 to 230 g) and their offspring, the newborn rats (1 day after birth, the body weight of 5.83–7,62 g), divided into four experimental groups. For the simulation of hemic hypoxia from the first day of pregnancy the females of the first series were daily for 21 days (duration of pregnancy) intraperitoneally administered the sodium nitrite NaNO2 solution at a dose of 5 mg/100 g weight (dose causing hypoxia of moderate severity) from the first day of pregnancy. Females of the second group (I.2) and their offspring did not get any correction. Animals of the third group (I.3) in 30 minutes after administration of sodium nitrite over a 21 day intragastrically through the probe received water solution of «Fenocor» (an antioxidant) at a dose of 2.5 ml/ kg together with 0.5 ml of water. Females of the fourth group (I.4) in 30 minutes after administration of sodium nitrite were intraperitoneally injected with «Cytoflavin» (antihypoxant) at the rate of 1.5 ml/100 g of body weight. It was revealed that the effect of hypoxic factors leads to a pronounced destruction of cardiomyocyte contractile proteins, which manifested itself in the form of a significant expression rate decrease compared to the control indices of the immunohistochemical staining index of the main component of the fibrillar apparatus — sarcomeric actin and cytoskeleton protein — desmin, respectively: after exposure to hemic hypoxia in females — by 27, 27 % and 40.00 %, in newborns — by 34.04 % and 37.04 % (p <0.001). A significant part of the cardiomyocytes of experimental animals shows an almost total disappearance of transverse striation due to lysis of myofibrils and the disappearance of Z-bands of cardiomyocytes. Hypoxic conditions are accompanied by the development of arrhythmias mainly by extrasystoles followed by the ST segment elevation in both adult and newborn rats. The frequency of registration of arrhythmias during hemic hypoxia is 35 % for females and 46 % for newborn rats. The use of experimental correctors demonstrated the cytoprotective properties contributed to the preservation of rat myocardial structure under conditions of histotoxic hypoxia.
Keywords: myocardium, hypoxia, cardiomyocytes, pregnancy, antihypoxants, rats.
Introduction
According to the modern pathophysiological concepts chronic hypoxia is considered to be one of the leading etiological factors of cardiovascular diseases, which can be caused by various physical, chemical and biological factors. The damaging impact of hypoxia is aggravated by the snowballing accumulation of oxidized substrates resulting in the generation of oxygen reactive species (ROS) that in turn provoke the progressive disruption of the respiratory chain exacerbating the energy shortage in cells accompanied by destruction of cell membranes [Anzell AR, et al.,2018].
There is no any doubt that the mankind impact on the environment has greatly increased due to the non-stop progress of science and technology, the accelerated processes of industrialization and urbanization. Traditionally so-called anthropogenic environmental factors are arising from human activities. By most of them we assume the various types of environmental pollutants since they have quite distinct manifestations of the changes in the functional state of the organism. It is well known that many xenobiotics possess hypoxic and prooxidant properties. In particular, the increasing environmental contamination by nitric compounds appears to be quite a big threat towards human health [Adamkiewicz G, et al., 2004]. Nitrate concentrations in plants vary from a few mg up to thousands of mg and depend on many factors, among which the most determining one stands for the increase of nitrates concentration in the soil due to the intensification of the natural nitrification process or due to the uncontrolled application of nitrogen fertilizers. In many countries the last factor is prevailing. It was also noted that some pesticides and other toxic compounds, disrupting the metabolism in plants, increase the accumulation of nitrates, for example, as the herbicide 2,4-D up to 10–20 times.
In the most of developed and developing countries the leading place in the structure of morbidity and mortality rate belongs to cases related to the development of diseases of the circulatory system, which are prevalent among different forms of coronary heart disease [Benjamin EJ, et al. 2017]. In recent years numerous researches have been done to reveal the peculiarities of the cellular protein molecules reaction upon various damaging factors, since the structural proteins of cardiomyocytes are the main part of cytoskeleton and participate in linkage of the cell membrane with the organelles [Bar H,et al.,2004]. New data, devoted to the study of proteins of the cytoskeleton of cardiomyocytes, will provide an opportunity to expand the understanding of the mechanisms underlying in the structural damage of cardiomyocytes during acute myocardial ischemia.
A special place in the study of hypoxic effects undoubtedly lies in the field of perinatal hypoxia [Patterson AJ, et al., 2010]. In the current data is present quite large amount of information on the influence of acute antenatal hypoxia of the final trimester of pregnancy on the histogenesis of the fetal myocardium associated with the hypoxic and functional consequences of these effects in the neonatal heart. However, the impacts of long-term effects of various chemical and physical hypoxic factors during perinatal period dealt is still not fully covered [Flamant-Hulin M, et al., 2010].
So, hypoxic damage to the myocardium is an undeniable reality of the cardiological practice. Nowadays we have clarified many mechanisms of hypoxic myocardial damage, but in-depth studies of the morphological substrate providing the symptoms of hypoxic myocardial damage in adults and children are scarce and rather contradictory [Costa M, et al., 2004]. Thus, the development of novel cardioprotection strategies requires a deeper understanding of the morphological aspects which occur during the hypoxic changes in the heart tissue [Bhatti JS, et al., 2017].
An innovative approach to pharmacotherapy of hypoxic damage is the development of relatively new class of pharmaceutical drugs — the so-called antihypoxants which are able to partially neutralize or even eliminate the pathogenic hypoxic effects by maintaining at least minimal energy balance in the cell, especially in the early postnatal period [Baczkó I, et al., 2015; Ravens U, et al.,2013]. An important task for the modern science is to provide the evaluation of the pharmacological safety of the official and little known antihypoxants along with the benefit and risk analysis of these means for the prevention of perinatal pathology [Mink PJ, et al., 2007; O Byrne DJ, et al., 2002].
For clarification and understanding of the mechanisms and morphogenesis of hypoxic heart damage the only possible solution is the experimental simulation of this condition in laboratory animals. Such experiments allow us to estimate the morphodynamics of the pathological process by the means of modern informative methods of morphological research, which are not always applicable in clinical practice.
The aim of this research was to conduct quantitative and comparative assessment of the expression of the main structural proteins of the contractile apparatus and the cytoskeleton of the contractile cardiomyocytes of mature and newborn rats exposed to hemic hypoxia.
Material and methods
The research was carried out on mature female Wistar rats (6–6,5 months old, body weight 200 to 230 g) and their offspring, the newborn rats (1 day after birth, the body weight of 5.83–7,62 g). For induction of pregnancy virgin females were housed with sexually mature males with a ratio of two males to four females. The period of pregnancy was estimated from the moment of the detection of spermatozoa in the vaginal smear the morning (1st day of pregnancy).
According to the intended aim the experimental animals were divided into four groups:
— The 1st group was the control group, which consisted of intact animals;
— 2nd group of experiments was created for the simulation of pure hypoxic state without any correction;
— 3rd group of experiments was created for the simulation of hypoxic states combined with the introduction of an antioxidant;
— The 4th group of experiments consisted of animals exposed to hypoxia on the background of antihypoxant correction.
The number of intact animals: females — 6 in newborn rats — 6. The number of animals in each experimental group: females — 11, newborn rats — 15.
For the simulation of hemic hypoxia from the first day of pregnancy the females of the first series for 21 days (duration of pregnancy) daily intraperitoneally were injected sodium nitrite NaNO2 at a dose of 5 mg/100 g weight (dose causing hypoxia of moderate severity). Females of the second group (I.2) and their offspring did not receive any correction. Animals of the third group (I.3) in 30 minutes after administration of sodium nitrite over a 21 day through the gastric probe received the water solution «Fenocor» (antioxidant, LLC «RESSFUD», Yalta, the patent for useful model RU 150139 U1) at a dose of 2.5 ml/ kg together with 0.5 ml of water [Zadnipryany IV et al, 2017]. Females of the fourth group (I.4) in 30 minutes after administration of sodium nitrite were intraperitoneally injected with «Cytoflavin» (antihypoxant LLC «stpf «POLYSAN», Russia) at the rate of 1.5 ml/100 g of body weight [Zarubina IV., et al., 2012]. Their pups did not received any medication after birth, because it was assumed that the correctors could penetrate their body via placenta. Control animals of this series received saline injections.
Animals were housed in vivarium according to the rules and International recommendations of the European Convention for the Protection of Animals (1997). Experimental studies were carried out in accordance with the ethical requirements for working with experimental animals, Order of the USSR Ministry of Health No. 755 of August 12, 1987 “Rules for Working with Experimental Animals”, Federal Law “On the Protection of Animals Against Cruel Treatment” of January 1, 1997, Order of the Ministry of Health Of the Russian Federation No. 267 dated June 19, 2003 “On the Approval of Laboratory Practice Rules” and approved by the local ethical committee of the S. I. Georgievsky Medical Academy of V. I. Vernadsky Crimea Federal University.
After sacrificing the animals under ether anesthesia left ventricular myocardium fragments were fixed for 24 hours in 10 % formalin solution, then the material was degreased and dehydrated in increasing alcohols, embedded in paraffin, and histological preparations were made with sections about 5 microns. For immunohistochemical studies, the prewashed myocardium was fixed in a 10 % buffered formalin solution with further wiring and manufacturing of paraffin blocks. The immunohistochemical (IHC) reactions were carried out on sections with a thickness of 3–4 μm using the double peroxidase-antiperoxidase method in accordance with the protocols of Termo Scientific (USA) using the Leica Biosystems (Novocastra) and DAB Chromogen imaging systems and subsequent nuclear staining with hematoxylin [Dabbs DJ., 2010]. Quantification of the expression intensity of sarcomeric actin (clone MA1–21597, Thermo Scientific) and desmin (clone PA1–27003, Thermo Scientific) was evaluated by the Posistive Pixel count v.9 software protocol by the formula count:
where Nsr is the relative number of dark brown positive pixels;
Nsp is the total number of dark brown positive pixels;
Nwp is the number of slightly positive pixels;
Np is the total number of positive pixels in a photo with an area of 44839.31 μm2 at 400x magnification.
The obtained microslides were examined by Olympus CX-31 microscope (Japan). Morphometric measurements were performed at a magnification of 400 using the licensed program J Image.
The electrocardiogram (ECG) was recorded in mature and newborn rats using the Reokom computer rheographic complex (Ukraine), and the ST segment elevation and the incidence of arrhythmias were estimated by complex software.
Statistical data processing was calculated on the licensed software Statistica 10.0. When analyzing the results of IHC research methods, we estimated the arithmetic average for the whole group, the standard deviation, the average error, the coefficient of variation, the deviation of the value in the experiment from the value in the control in percent. The obtained quantitative data were subjected to a preliminary analysis for compliance of the distribution of characters with the normal law. Comparisons between groups were determined by a one-way ANOVA with a post-hoc Student t-test (SPSS Inc., Chicago, IL). Probabilities of p<0.05 were considered statistically significant.
Results
The main task of a morphological study in assessing the contractile function of the myocardium against hypoxia is the detection of a genuine substrate of myocardial contraction, i.e. fibrillar apparatus of cardiac fibers. Sarcomeric actin (SA) is the important structural protein of myofibrils, providing the contractile function of myofibrils and forming thin filaments of myofibrils. During the exposure to hemic hypoxia without correction a significant decrease in the immunostaining index (IMO) of sarcomeric actin in rat cardiomyocytes (CMC) was revealed: by 27.27 % in females and by 34.04 % in newborns (p <0.05) (table 1).
Table 1
Immunostaining index (IMI) of sarcomeric actin and desmin of rats exposed to hemic hypoxia on the background of correction (M ± m)
|
Groups |
IMI |
||
|
FEMALES |
PUPS |
||
|
I1 |
SA |
0,77±0,05 |
0,47±0,06 |
|
Desmin |
0,45±0,02 |
0,27±0,06 |
|
|
I2 |
SA |
0,56±0,04* |
0,31±0,07* |
|
Desmin |
0,27±0,04* |
0,34±0,05* |
|
|
I3 |
SA |
0,60±0,01* |
0,27±0,06* |
|
Desmin |
0,36±0,01*/** |
0,20±0,03* |
|
|
I4 |
SA |
0,66±0,07* |
0,39±0,08** |
|
Desmin |
0,39±0,03** |
0,23±0,16* |
|
Note: *- р < 0,05 control significance.
** — р < 0,05 significance with I.2.
The microscopy of myocardial sections had revealed the IHC reaction was usually absent in the center of the cardiac muscle fiber, which can be probably explained due to the effect of prolonged ischemia, more vivid in the left ventricle (Fig. 1.a). In all samples it was noted the lack of expression of this protein in myofibrils of a significant number of cardiomyocytes and that was especially well demonstrated on the myocardial cross sections (Fig. 1.b).
The evaluation of desmin expression had revealed that uneven staining of sarcomeres and insertion discs of cardiomyocytes was observed mainly in the ischemic zone. Transverse striation in some cells was totally absent. Along with those unstained cells the enlarged hypertrophied cardiomyocytes with areas of increased transverse striation were found (Figure 1.c). A decrease in the immunostaining index (IMO) of desmin in CMC was found to be 40.0 % in females and 37.04 % in newborns (p <0.05), which evidenced for the myocardial dystrophic changes (Figure 1.d).
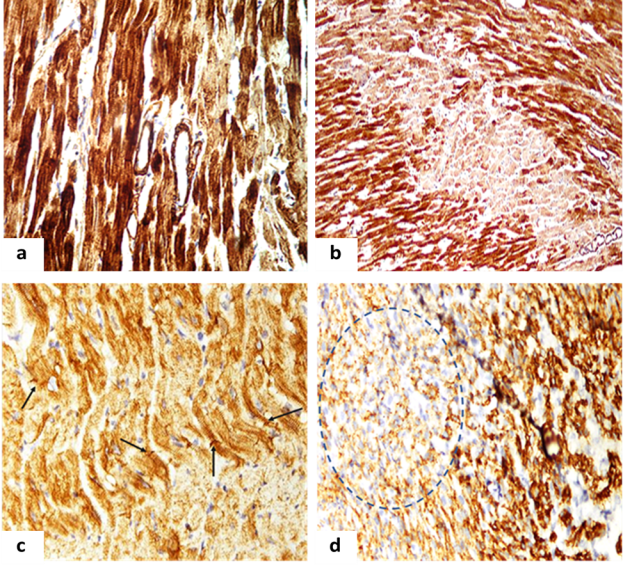
Fig. 1. Myocardium of adult and newborn rats on the background of hemic hypoxia:
a. The myocardium of the left ventricle of female rats with reduced expression of sarcomere actin. IHC x 400.
b. The left ventricular myocardium of newborn rats with reduced expression of sarcomere actin. IHC x 400.
c. Myocardium of the left ventricle of the female rat. Discomposition and disappearance desmin Z-bands (arrows). IHC x 400.
d — Zone with diminished expression of desmin (desminfree zone) in the myocardium of newborn rats (dotted line). IHC x 400
On the background of experimental cardioprotection a decrease in the expression of SA in rat myocardium was rather diffuse, it was noted zones of moderate expression were combined with high expression areas, apparently due to the development of compensatory hypertrophy of relatively intact CMC.
The infusion of grape polyphenols made IMO of SA to increase significantly in comparison to the previous group of animals which did not received antihypoxic correction, in females — by 6.67 %, in newborn by 8.82 % (p>0.05). Similarly, IMO of desmin was increased in females up to 25.00 % (p<0.05) and up to 15.00 % (p>0.05) in newborn rats, but still remained below normal control value of 20.00 % and of 25.92 % (p<0.05), respectively.
With the introduction of polysubstrate antihypoxant which contained succinic acid in comparison with the group I.2 without correction IMO of SA was increased in females by 15.15 % and similarly by 20.51 % (p<0.05) in newborns, thus it was still remaining below the reference values of 14.28 % and of 17.02 % (p>0.05), respectively (Fig.2.a, 2.c).
IMO of desmin was increased in females and their offspring by 30.77 % and 26,09 % (p<0.05) in comparison with group without correction and by 7.69 % and 13.04 % (p<0.05) in comparison with the previous group, but remained below control values by 13.33 % and 14,18 % (p>0.05) (figure 2.b, 2d). And in some CMC noted the overexpression of this protein that may have been due to their compensatory hypertrophy. It is obvious that a rather high content of structural proteins due to cytoprotective effect of the correctors, even on the background of hypoxia will allow the CMC to maintain a relatively stable state resulting mechanical deformation in the process of contraction and relaxation of muscle fibers.
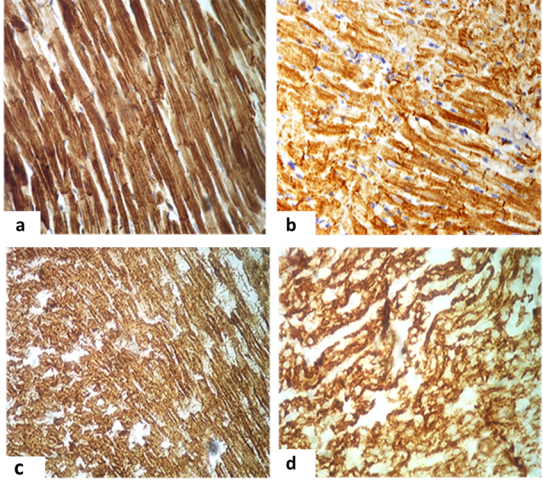
Fig. 2. Cardiomyocytes of the left ventricle of females and newborn rats received antihypoxant correction:
a. Uniform intense expression of sarcomeric actin in the sarcoplasm of most CMCs in female. IHC x 400.
b. Ordered expression of desmin in the region of Z-bands and insertion discs in female. IHC x 400.
c. Intensive expression of sarcomeric actin in the sarcoplasm of most CMCs, the appearance of transverse striation of sarcomeres in newborn. IHC x 400.
d. Intense expression of desmin, completely filling the sarcoplasm of cells in newborn. IHC x 400
In all experimental groups of animals the modeling of hemic hypoxia caused significant changes in the functional activity of the myocardium, reflected on the ECG. The most significant sign of hypoxic damage was the rise of the ST segment above the contour, which reflects ischemia. The value of the characteristic ST elevation in the form of a “cat's” back was 1.53 ± 0.06 mm in females and 1.23 ± 0.04 mm in newborn rats. The frequency of registration of arrhythmias, mainly of the type of atrial and ventricular extrasystoles, in modeling hemic hypoxia was 35 % of cases for females and 46 % of cases for newborn rats. So, when registering an ECG in newborn rats of the first group, along with bradycardia (less than 330 beats / min) in some, “volleys” of supraventricular extrasystole were revealed against the background of severe bradycardia in other animals of this group.
The introduction of the antioxidant was accompanied by some improvements in ECG parameters. The average ST-segment elevation was 1.31 ± 0.18 mm in females and 0.75 ± 0.14 mm in newborns. In this group, at the end of the experiment, arrhythmia was registered in 21 % of females and 32 % of newborns. Administration of an antihypoxant led to a decrease in the number of rats with arrhythmias up to 11 % in females and 23 % in newborns. The recorded ST segment lift was 1.02 ± 0.07 mm and 0.48 ± 0.13 mm, respectively. Preventive administration of the drug to females also led to a decrease in heterotopic rhythm disturbances in their offspring: despite the presence of frequent bradycardia (350–450 beats / min), extrasystoles were practically not much recorded in this group.
Discussion
The research of rather poorly studied cytoskeleton proteins of CMC can greatly enhance the understanding of the mechanisms underlying in mechanism of their structural damage and violation of their contractile properties under hypoxia. The desmin protein provides cross fitting of myofibrils maintaining the constant length of the sarcomere before and after cell contraction. Its physical properties allow the cardiomyocytes to maintain a stable state when mechanical deformation occurs in the process of contraction and relaxation of muscle fibers during cardiac systole and diastole. In addition, desmin provides a little-studied interaction of myofibrils with organelles CMC and largely determines their spatial location in the cell. Desmin is localized, usually in interfibrillar space on the periphery of Z-bands and also in the locations of the intercalated discs, i.e. in the region of the desmosomes [Bar H, et al., 2004]. Immunochemical staining of sections on the background of hemic hypoxia in the ischemic zone of the myocardium has revealed the decreased the number of intercalated discs with frequently observed deformed discs in the form of zigzag sections or «broken stripes». In areas of myocardium where the intercalated disks were destroyed, we observed the elongation of the sarcomeres CMC. With the introduction of cobalt chloride in some CMC there were noted blurring transverse striation, the cytoplasm had a lumpy or granular structure. In a separate, probably compensatory exaggerated, CMC was revealed an increased expression of desmin. However, in many CMC transverse striations were hardly discernable or have been preserved only in the form of separate foci. In the ischemic zone remained isolated inserted. At newborn rats in both series was marked by extensive zones of «desmin-free» of cardiomyocytes.
Obviously, the exposure to nitric xenobiotic resulted in the increased content of calcium ions and hydrogen in CMC, in turn, this lead to the activation of calcium-dependent proteases, which caused lysis of desmin. Thus it is necessary to take into account the role of desmin in the mitochondrial functioning of the CMC, as it communicates with the mitochondria sarcomeres of the cell. This allows to provide the link between the reduction and the emerging need for new energy supply. Obviously, desmin is an important regulator of the level of energy and oxygen balance CMC. Loss of desmin probably could serve as the another morphological substrate of the arrhythmia [Savchenko S. V.,2015]
The result of the oxidation of functional groups of biologically active substances can be degradation of structural proteins and lipids of cell membranes, in particular the observed lysis of mitochondria and myofibrils, as well as modification of nucleic acids. In this regard, the obvious importance of experimental studies aimed at investigating the metabolic effects of different types of antioxidants, antihypoxants, membraneprotective in conditions of acute hypoxia [Vaisman N, et al., 2015; Wightman JD, et al., 2015].
Antioxidant activity of polyphenolic concentrates of grapes is provided due to the presence of flavonoid (anthocyanins, quercetin, epicatechin, tannins) and neoflavonoids (lilac and Gallic acid, resveratrol) components [Kubyshkin AV, et al., 2017]. Mechanism of antioxidant activity of plant polyphenols is associated with their ability to chelation of ions of metals of variable valency, at least, this mechanism occurs in the case of induced lipid peroxidation [Sano A, et al., 2007; Li H, et al., 2012].
«Cytoflavin» is a complex polysubstrate antihypoxant and antioxidant drug, which represents a pharmacologically reasonable selection of drugs, allowing to achieve the greatest possible positive impact on the metabolism due to the summation effects of its components in the conditions experienced by the organism hypoxia and oxidative stress [Zarubina IV., et al., 2012].
It is obvious that in the conditions of development of hemic (anemic) hypoxia greatest efficiency should be provided by antihypoxants with powerful regenerative and antioxidant properties, since the leadin pathogenic factor of nitric hypoxia is the accumulation of methemoglobin due to the the oxidation of haemoglobin by the superoxide anion radical. Antioxidant protection can prevent a rapid increase in the concentration of methemoglobin, thereby providing a systemic protective effect. The application of antihypoxants along with the above-described arrangements performs additional reactivation of succinate dehydrogenase of Krebs cycle [Zhang, J, et al., 2018].
Conclusion
Described immunohistochemical studies of contractile proteins and proteins of the cytoskeleton of the myocardium can be effectively applied in the diagnosis of cardiomyopathies as serving as the definition tool for the features of its course and an unfavorable outcome, predicting the likelihood of life-threatening arrhythmias in strategies for personalized medicine. The obtained morphological data is also partially explaining the formation of the so-called hidden heart failure of newborns when indicators of blood circulation practically do not change, but a number of indices of diastolic function indicate a primary lesion of the myocardium. This is extremely life-threatening state as it can the suddenly exacerbate into rapid heart failure that is caused by low contractile reserve of the left ventricle and the immaturity of the regulatory mechanisms of fetal myocardium.
The revealed by immunohistochemical methods structural changes of the myocardium can be predictors of heart failure, which requires the adequate cardioprotection. The use of «Fenocor», which demonstrated cytoprotective properties, had contributed to the preservation of myocardial structure of rats in conditions of histotoxic hypoxia. The structure of the myocardium observed in newborn rats on the background of antenatal preconditioning generally reflected the tendency for limitation of cardiac damage manifested as morphological preservation myofibrils and Z-bands.
References:
- Adamkiewicz G, Ebelt S, Syring M, et al. Association between air pollution exposure and exhaled nitric oxide in an elderly population. Thorax. 2004;59:204e9
- Anzell AR, Maizy R, Przyklenk K, et al. Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol. Neurobiol. 2018; 55: 2547–2564
- Baczkó I, Light PE. Resveratrol and derivatives for the treatment of atrial fibrillation. Ann. N. Y. Acad. Sci. 2015; 1348; 68–74.
- Bar H, Strelkov S, Sjöberg G, Aebi U, Herrmann Н. The biology of desmin filaments: how do mutations affect their structure, assembly, and organization? Journal of Structural Biology. 2004;148: 137–152.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update a report from the American Heart Association. Circulation. 2017; E146–E603 [PubMedID: 28874428].
- Bhatti J. S., Bhati G. K., Reddy P. H. Mitochondrial dysfunction and oxidative stress in metabolic disorders — a step towards mitochondria based therapeutic strategies. BBA-Mol. Basis. Dis. 2017; 1863: 1066–1077 [PubMedID: 27836629].
- Costa M, Escaleria A, Cataldo A, Oliveria F, Mermelstein C. Desmin: molecular interactions and putative functions of the muscle intermediate filament protein. Brazilian Journal of Medical and Biological Research. 2004; 37 (12): 1819–1830.
- Dabbs DJ. Diagnostic immunohistochemistry. 3rd ed. Elsevier Inc., 2010. 941 р.
- Flamant-Hulin M, Caillaud D, Sacco P, et al. Air pollution and increased levels of fractional exhaled nitric oxide in children with no history of airway damage. J Toxicol Environ Health A 2010; 73(272):e83.
- Kubyshkin AV, Avidzba AM, Borisyuk VS, et al. Polyphenols of red grapes in wine and concentrates for use in rehabilitation technologies. Agricultural Biology. 2017; 52(3): 622–630.
- Li H, Xia N, Förstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide. 2012; 26:102–110.
- Mink PJ, Scrafford CG, Barraj LM, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85(3):895–909.
- O’Byrne DJ, Devaraj S, Grundy SM, et al. Comparison of the antioxidant effects of Concord grape juice flavonoids and α-tocopherol on markers of oxidative stress in healthy adults. Am. J. Clin. Nutr. 2002; 76:1367–1374.
- Patterson AJ, Chen M, Xue Q, et al. Chronic prenatal hypoxia induces epigenetic programming of PKCε gene expression in rat hearts. Circulation Res. 2010;107:365–73.
- Ravens U, Poulet C, Wettwer E, Knaut M. Atrial selectivity of antiarrhythmic drugs. J. Physiol. 2013; 591:4087–4097.
- Savchenko SV. Patho-morphological research in medico-legal practice at the present stage. Bulletin of Forensic Medicine. 2015; 4 (2):21–24.
- Vaisman N, Niv E. Daily consumption of red grape cell powder in a dietary dose improves cardiovascular parameters: A double blind, placebo-controlled, randomized study. Int. J. Food Sci. Nutr. 2015; 66:342–349.
- Wightman JD, Heuberger RA. Effect of grape and other berries on cardiovascular health. J. Sci. Food Agric. 2015; 95:1584–1597.
- Zhang J, Wang Y, Miller JH, Day MM, Munger JC, Brookes PS. Accumulation of succinate in cardiac ischemia primarily occurs via canonical Krebs cycle activity. Cell Rep. 2018; 23:2617–2628.
- Zadnipryany IV, Tretiakova OS, Kubyshkin AV, Sataieva TP. Protective effect of grapes polyphenol concentrate “Fenokor” in terms of hypoxic myocardial injury. Bulletin of Siberian Medicine. 2017; 16 (3):34–42.
- Zarubina IV, Lukk MV, Shabanov PD. Antihypoxic and antioxidant effects of exogenous succinic acid and aminothiol succinate-containing antihypoxants. Bull Exp BiolMed. 2012; 153 (3):336–339.







