Introduction
Aortoiliac occlusive disease (AIOD) is a specific type of peripheral artery disease that impacts the infrarenal aorta and iliac arteries, leading to obstruction of blood flow to distal organs due to narrowed arterial lumens or plaque embolization. This condition can manifest a broad spectrum of symptoms, ranging from asymptomatic cases to limb-threatening emergencies. In many instances, collateral circulation may adequately manage symptoms without the need for surgical intervention. However, obstructive lesions are frequently located in the infrarenal aorta, common iliac artery, internal iliac (hypogastric) artery, external iliac artery, or combinations of these vessels.
By identifying the various risk factors associated with AIOD, clinicians can implement medical therapies aimed at alleviating symptoms and enhancing patient survival. The introduction of prosthetic graft materials in the 1960s transformed the surgical management of AIOD, providing an effective option for patients with significant occlusions in the aorta and iliac arteries. This review will explore the pathophysiology, clinical manifestations, diagnostic methods, and current management strategies, with an emphasis on surgical and endovascular interventions designed to improve outcomes for patients with AIOD.
Etiology
AIOD is a variant of peripheral artery disease (PAD) that specifically affects the lower aorta and iliac arteries. Like PAD, AIOD is primarily caused by progressive atherosclerosis, which involves the accumulation of plaques made up of lipids, cholesterol, calcium, and other substances within the arterial walls. These plaques can impede blood flow to the lower extremities, pelvis, and abdominal organs, resulting in stenosis and potential occlusion of the infrarenal aorta as well as the common, internal (hypogastric), or external iliac arteries.
Several factors contribute to the development of atherosclerosis and AIOD, including diabetes, hyperhomocysteinemia, hypertension, hyperlipidemia, and tobacco use. Smoking is particularly detrimental as it accelerates plaque formation through inflammation and endothelial dysfunction. Other significant risk factors include age, family history, ethnicity, and sex. Conditions like diabetes and hypertension increase vascular stress and heighten the risk of developing atherosclerotic plaques. Additionally, hyperlipidemia—especially high levels of low-density lipoprotein (LDL) cholesterol—plays a direct role in arterial plaque accumulation.
Epidemiology
Determining the precise prevalence of aortoiliac occlusive disease and peripheral artery disease as a whole is challenging, as many patients remain asymptomatic. Estimates suggest prevalence rates ranging from 3.56 % to over 14 % in the general population. Studies indicate a higher prevalence among older individuals, with estimates of 14 %-20 % in those over 70 years old and 23 % in those over 80. The condition is more prevalent in males and non-Hispanic black populations, as well as among individuals with the aforementioned risk factors.
Pathophysiology
AIOD progressively impedes blood flow in the infrarenal aorta and iliac arteries, primarily due to atherosclerosis. The underlying pathophysiology of AIOD begins with endothelial injury, triggering a series of events that lead to the formation of atherosclerotic plaques.
Various risk factors, including diabetes, hypertension, hyperlipidemia, and smoking, inflict damage on endothelial cells through multiple mechanisms. These factors promote endothelial dysfunction by increasing the expression of leukocyte adhesion molecules, elevating inflammatory cytokines, and decreasing nitric oxide production. Consequently, atherosclerosis develops as lipids, macrophages, and smooth muscle cells accumulate within the arterial walls. Over time, these deposits merge into fibrous plaques that may calcify, further narrowing the arterial lumen and restricting blood flow to distal organs. Additionally, heightened production of endothelial vasoconstrictors, superoxide anions, and prothrombotic factors worsens vascular dysfunction. As plaques progress, the structural integrity of underlying vascular smooth muscle diminishes while elevated serum-free fatty acids and insulin levels further impair cell function.
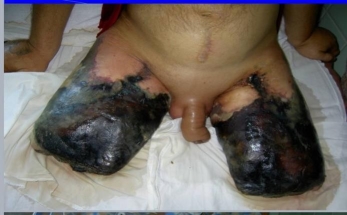
In AIOD cases, this narrowing typically occurs in the infrarenal aorta or common and external iliac arteries. Initially, collateral circulation may compensate for reduced blood flow, allowing some patients to remain asymptomatic. However, as atherosclerosis advances and these compensatory mechanisms fail, symptoms such as claudication, rest pain, and critical limb ischemia can arise. Moreover, plaque rupture or dislodgment may lead to distal embolization and worsen ischemic symptoms. If left untreated, this progressive ischemia can result in tissue loss and potentially necessitate limb amputation. Nonatherosclerotic conditions like large vessel vasculitis—such as Takayasu arteritis—can also cause similar vascular occlusion and ischemic effects.
The risk factors associated with AIOD lead to endothelial cell injury through various mechanisms. Endothelial injury and dysfunction play a crucial role in the development of atherosclerosis and its complications through several pathways:
History and Physical Examination
Patients typically present with cramping pain during and after physical activity, which is alleviated by rest—a condition known as claudication. A comprehensive history and physical examination are essential for assessing disease severity and differentiating it from other conditions. Generally, cramping in more proximal muscles indicates a greater degree of stenosis. Some patients may exhibit a triad of symptoms: buttock claudication, erectile dysfunction, and absent femoral pulses, collectively referred to as Leriche syndrome, first described by Dr. René Leriche, a distinguished French surgeon.
Patients may also seek emergency care due to complications arising from severe stenosis or acute embolic events that lead to chronic limb-threatening ischemia (CLTI). The diagnostic criteria for CLTI include rest pain, gangrene, or lower limb ulceration persisting for over two weeks in the context of peripheral artery disease.
Diagnosis
Evaluating Arterial Insufficiency and Obstruction Disease (AIOD) requires a thorough approach that combines clinical history, physical examination, and diagnostic tests to ascertain the presence and severity of arterial blockages. In patients with multiple vascular risk factors, such as claudication and absent femoral pulses, diagnosis is often straightforward. However, some individuals may still have palpable pedal pulses at rest due to robust collateral circulation, highlighting the importance of detailed history-taking.
Laboratory
Laboratory assessments are crucial for identifying underlying risk factors associated with AIOD. Tests such as serum lipid profiles, hemoglobin A1c, lipoprotein A, and homocysteine levels can indicate conditions like hyperlipidemia and diabetes, which significantly contribute to atherosclerosis. In cases where thrombosis is suspected, prothrombin time (PT), activated partial thromboplastin time (aPTT), and platelet counts are essential. Additional tests may be warranted for further clarification, including anticardiolipin antibodies, antithrombin III levels, factor II (prothrombin) C-20210a mutation analysis, factor V Leiden testing, and proteins C and S evaluation. Given its link to coronary artery disease, an electrocardiogram (ECG) should also be included in the assessment.
Ankle-Brachial Index
The ankle-brachial index (ABI) is a primary screening tool endorsed by the American Heart Association (AHA) and the United States Preventive Services Task Force for diagnosing arterial disease due to its affordability, reliability, and noninvasive nature. An ABI score below 0.9 indicates arterial occlusion; however, a normal resting ABI can sometimes be misleading because of collateral circulation. Performing an ABI after graded exercise treadmill testing can help identify occlusive disease in patients exhibiting symptoms of AIOD.
Imaging Studies
– Pulse Volume Recordings: These recordings are recommended alongside ABI as part of the initial evaluation process. They assess changes in blood volume within the limb to provide insights into the extent of arterial blockages.
– Doppler Ultrasonography: Duplex ultrasound is a widely used noninvasive method for detecting blood flow abnormalities and locating stenotic or occlusive areas in the aorta and iliac arteries. This imaging technique aids in determining whether further imaging is necessary.
– Computed Tomography Angiography (CTA): CTA provides detailed three-dimensional images of the aorta and iliac arteries to identify blockage locations and severity. This modality is particularly beneficial for planning interventions like endovascular procedures or surgical bypass.
– Digital Subtraction Angiography (DSA): Often considered the gold standard for detailed vascular imaging, DSA is typically reserved for cases where endovascular intervention is planned since it offers real-time visualization along with treatment opportunities.
Treatment and Management
There has been a notable evolution in the management of peripheral artery disease (PAD), with angioplasty and stenting now recognized as the primary treatment options for most patients suffering from aortoiliac, renal, subclavian, and coronary occlusive diseases. The onset and severity of symptoms play a critical role in determining the approach to managing aortoiliac occlusive disease (AIOD). In cases of chronic limb-threatening ischemia (CLTI), timely intervention is essential to avert further tissue necrosis and gangrene. The «PLAN» framework—encompassing patient risk, limb staging, and anatomic pattern—can effectively stage the disease and inform treatment decisions.
Medical Management
For nonacute cases, particularly in patients deemed poor surgical candidates, medical management is an option. Key strategies include outpatient optimization and management of conditions such as diabetes, hyperlipidemia, hypertension, prothrombotic states, and tobacco cessation. A healthy diet and regular exercise are also recommended. Supervised exercise programs have been shown to significantly enhance walking distances, potentially increasing them by 180 % to 340 %.
Claudication symptoms can be managed with medications like cilostazol or pentoxifylline. Cilostazol, a phosphodiesterase III inhibitor, may improve graft patency and reduce the risk of stenosis following surgical procedures. Pentoxifylline, a methylxanthine derivative, also alleviates symptoms but is generally considered less effective than cilostazol.
Antithrombotic Agents
The CAPRIE trial demonstrated that clopidogrel offers superior antiplatelet management compared to aspirin, leading to reduced mortality rates from ischemic strokes, myocardial infarctions, and other vascular-related issues. While other antiplatelet agents have not undergone direct comparison studies, dual antiplatelet therapy is not recommended as a primary treatment. Vorapaxar, which acts as an antagonist of the protease-activated receptor (PAR-1), has shown promise in improving outcomes for acute limb ischemia when used alongside antiplatelet therapies. Vitamin K antagonists have not demonstrated improved outcomes either alone or in combination with aspirin. Additionally, subset analyses of patients with peripheral artery disease indicate that combining rivaroxaban with aspirin can lower the incidence of major adverse limb events.
Surgical and Endovascular Treatment
Revascularization techniques—both surgical and endovascular—are crucial for treating AIOD, providing various options to restore blood flow and relieve symptoms such as claudication and critical limb ischemia.
Surgical Revascularization Techniques
Open surgical revascularization involves bypassing stenotic or occluded areas using vascular conduits. The choice of bypass depends on the extent and location of the blockage, as well as the patient's comorbidities and surgical risk profile. Several surgical options include:
– Aortoiliac Bypass Graft: This procedure requires extensive exposure and aortic clamping, making it more invasive but effective. The surgeon accesses the infrarenal and iliac arteries through an abdominal incision, utilizing a Y-shaped polytetrafluoroethylene (PTFE) graft to bypass the blockage. This method has demonstrated excellent long-term outcomes, with 5- year patency rates exceeding 90 %.
– Aortobifemoral Bypass (AFB): AFB is a frequently employed surgical option that uses a PTFE graft to bypass the aorta to the femoral arteries. It is particularly effective for extensive occlusive disease, achieving 5- and 10-year patency rates of 85 % to 90 % and 75 % to 80 %, respectively.
– Axillofemoral Bypass: This extraanatomic bypass is suitable for patients with contraindications to aortoiliac or AFB bypass due to significant comorbidities. It involves tunneling a PTFE graft from the axillary artery to the femoral arteries, providing an alternative blood flow route. Although it is less durable than an AFB bypass, it offers acceptable patency rates and can be performed in higher-risk patients.
– Femorofemoral and Axillopopliteal Bypass: These are variations of the axillofemoral bypass, typically employed in specific cases involving unilateral disease or challenging anatomical considerations.
Endovascular Revascularization Techniques
Endovascular procedures are less invasive compared to open surgery and utilize catheter- based methods to restore blood flow:
– Percutaneous Transluminal Angioplasty (PTA): This technique involves the insertion of an inflatable balloon catheter, guided by a wire, into the stenotic or occluded vessel. The balloon is inflated to compress atherosclerotic plaque against the arterial wall, effectively widening the lumen. PTA achieves nearly 90 % primary patency at one year, with primary assisted and secondary patency rates reaching 92.3 %. This procedure can be performed with or without the placement of a stent, and studies indicate that covered stents yield better outcomes than bare-metal stents. Additionally, endovascular treatments have demonstrated effectiveness in addressing challenging calcified lesions.
– Thromboendarterectomy (TEA): TEA entails the surgical removal of atherosclerotic plaque directly from the arterial wall and is typically conducted alongside PTA. While this procedure is more invasive than PTA, it can be particularly effective for patients with localized occlusive disease.
Differential Diagnosis
Vascular Conditions:
– Arterial aneurysm
– Arterial dissection
– Embolism
– Giant cell arteritis (GCA)
– Takayasu arteritis
– Venous claudication Non-Vascular Conditions:
– Musculoskeletal pain
– Neurogenic claudication
Prognosis
Without intervention, the prognosis for aortoiliac occlusive disease (AIOD) is poor; however, the formation of self-compensating collateral circulation may enhance outcomes. More distal occlusions are linked to worse prognoses. Medical management can offer benefits, potentially delaying the need for surgical intervention or even eliminating it altogether.
After surgical intervention, the prognosis is more promising, with a 30-day mortality rate of 2–3 %. Aorto-bifemoral bypass (AFB) shows primary patency rates of 86.2 % at 5 years and 77.6 % at 10 years. Additionally, 10-year limb salvage rates and overall survival rates stand at 97.7 % and 91.7 %, respectively. Endovascular interventions demonstrate an even lower in- hospital mortality rate of 0.6 %, with primary patency rates of 96 % and 94 % at 1 and 2 years, respectively.
Complications
Untreated AIOD can lead to complications such as weakness, fatigue, impotence, and sexual dysfunction due to reduced blood flow. There is also an increased risk of heart failure, myocardial infarction, gangrene, and amputation in cases of unmanaged AIOD. Risks associated with surgical and endovascular treatments include graft thrombosis, wound infections, bleeding, and complications related to anesthesia.
Enhancing and outcomes.
Interprofessional communication and care coordination are vital in the treatment of AIOD, as each healthcare professional contributes unique expertise to the care plan. Pharmacists optimize medication regimens by managing anticoagulation and addressing risk factors like hyperlipidemia and hypertension. Effective collaboration among all team members, including physical therapists and dietitians, ensures a unified approach to managing patient risk factors and preventing disease progression. Regular interdisciplinary team meetings and the use of electronic health records to share current patient information enhance team performance, reduce medical errors, and promote a comprehensive strategy for managing AIOD, ultimately leading to improved patient-centered care and better long-term outcomes.
Effective management of aortoiliac occlusive disease (AIOD) necessitates a multidisciplinary approach to achieve optimal patient outcomes, enhance safety, and provide patient-centered care. It is essential for physicians, including vascular surgeons and interventional radiologists, to apply their specialized skills in diagnostic imaging, decision-making regarding medical versus surgical interventions, and performing procedures such as aortofemoral bypass or endovascular stenting. Advanced practitioners play a critical role in preoperative assessments, postoperative care, and patient education, ensuring that patients adhere to lifestyle changes and medical therapies. Nurses contribute by monitoring patients for signs of ischemia or complications, facilitating education, and conducting ongoing assessments to enable timely interventions, thereby improving patient safety and outcomes.
References:
- Kimyaghalam A, Fitzpatrick NJ, Khan YS. Aortoiliac Occlusive Disease. 2024 Oct 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 32644512.
- Wooten C, Hayat M, du Plessis M, Cesmebasi A, Koesterer M, Daly KP, Matusz P, Tubbs RS, Loukas M. Anatomical significance in aortoiliac occlusive disease. Clin Anat. 2014 Nov;27(8):1264–74. doi: 10.1002/ca.22444. Epub 2014 Jul 25. PMID: 25065617.
- Pescatori LC, Tacher V, Kobeiter H. The Use of Re-entry Devices in Aortoiliac Occlusive Disease. Front Cardiovasc Med. 2020 Aug 25;7:144. doi: 10.3389/fcvm.2020.00144. PMID: 33062643; PMCID: PMC7477292.
- Hope A, Wray A, Stephenson G. Initial Management and Recognition of Aortoiliac Occlusive Disease, A Case Report. J Educ Teach Emerg Med. 2022 Jan 15;7(1):V1-V4. doi: 10.21980/J87M0Z. PMID: 37483394; PMCID: PMC10358871.
- Brown KN, Gonzalez L. Leriche Syndrome(Archived). 2023 Feb 13. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 30855836.
- Ogunsanya NL, Milner R, Delaney CL, Puckridge P. Aortoiliac occlusive disease — a novel classification system based on anatomical segments and disease severity for management planning. Vascular. 2024 Oct;32(5):1005–1014. doi: 10.1177/17085381231166975. Epub 2023 Apr 11. PMID: 37040189.
- Shen CY, Zhang YB, Fang J, Qu CJ, Teng LQ, Li JL. [Advancement in endovascular therapy of aortoiliac occlusive disease]. Zhonghua Wai Ke Za Zhi. 2022 Feb 1;60(2):117–121. Chinese. doi: 10.3760/cma.j.cn112139–20211009–00480. PMID: 35012269.
- Mallory A, Giannopoulos S, Lee P, Kokkinidis DG, Armstrong EJ. Covered Stents for Endovascular Treatment of Aortoiliac Occlusive Disease: A Systematic Review and Meta- Analysis. Vasc Endovascular Surg. 2021 Aug;55(6):560–570. doi: 10.1177/15385744211010381. Epub 2021 Apr 27. PMID: 33902342.
- Sala F, Hassen-Khodja R, Declemy S, Bouillanne PJ, Haudebourg P, Batt M. Chirurgie laparoscopique aorto-iliaque pour lésions occlusives ou anévrismales [Laparoscopic aortoiliac surgery for occlusive disease and or aneurysms]. Ann Chir. 2003 Feb;128(1):4–10. French. doi: 10.1016/s0003–3944(02)00011–1. PMID: 12600322.
- Emrecan B, Onem G, Ocak E, Arslan M, Yagci B, Baltalarli A, Akdag B. Retroperitoneal approach via paramedian incision for aortoiliac occlusive disease. Tex Heart Inst J. 2010;37(1):70–4. PMID: 20200630; PMCID: PMC2829796.
- Martin D, Katz SG. Axillofemoral bypass for aortoiliac occlusive disease. Am J Surg. 2000 Aug;180(2):100–3. doi: 10.1016/s0002–9610(00)00426–8. PMID: 11044521.
- Scali ST, Schmit BM, Feezor RJ, Beck AW, Chang CK, Waterman AL, Berceli SA, Huber TS. Outcomes after redo aortobifemoral bypass for aortoiliac occlusive disease. J Vasc Surg. 2014 Aug;60(2):346–355.e1. doi: 10.1016/j.jvs.2014.02.002. Epub 2014 Mar 21. Erratum in: J Vasc Surg. 2015 May;61(5):1382. PMID: 24657290; PMCID: PMC4144400.
- Rodríguez SP, Sandoval F. Aortoiliac occlusive disease, a silent syndrome. BMJ Case Rep. 2019 Jul 15;12(7):e230770. doi: 10.1136/bcr-2019–230770. PMID: 31311790; PMCID: PMC6663198.
- Sen I, Stephen E, Agarwal S. Clinical profile of aortoiliac occlusive disease and outcomes of aortobifemoral bypass in India. J Vasc Surg. 2013 Feb;57(2 Suppl):20S-5S. doi: 10.1016/j.jvs.2012.06.113. PMID: 23336851.

