Introduction
Overview Edward Hughes, a British surgeon who treated patients with spontaneous or stress thrombosis of the subclavian or axillary vein, deep veins, and among the main veins that drain the body's upper extremities, was the first to describe Paget-Schroetter syndrome, also referred to as venous thoracic outlet syndrome or primary effort thrombosis, in 1949.
Dr. Hughes gave the syndrome the name «Paget-Schroetter» in honor of two medical professionals: Austrian internist and laryngologist Leopold von Schröetter, who postulated in 1884 that the clotting disorder was caused by repetitive musculoskeletal motion injuring the vein, and Sir James Paget, who documented a case of spontaneous thrombosis of the subclavian vein in a patient in 1875.
Three thoracic outlet syndromes, including Paget-Schroetter syndrome, arise when blood vessels
Etiology
Compression of subclavian vessels and the lower trunk of the brachial plexus (mainly occurs within the scalene triangle) due to:
– Physical trauma (e.g. hyperextension neck injuries)
– Repetitive motion of the abducted and externally rotated shoulder (e.g., tennis, baseball, swimming, repetitive throwing, carrying heavy objects overhead)
– Structural abnormalities
– Bones: anomalous cervical rib, collarbone fracture, exostoses of the first rib or collarbone
– Soft tissue: hypertrophic muscles in athletes and weight lifters, poor postureand obesity, hematoma, tumors (e.g., Pancoast tumor)
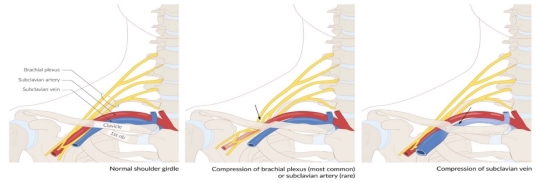
Fig. 1
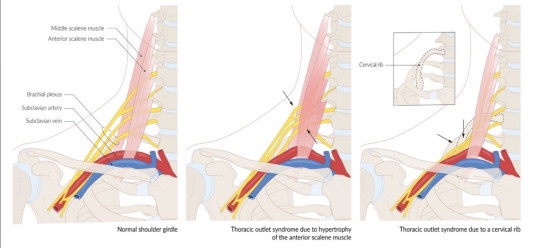
Fig. 2
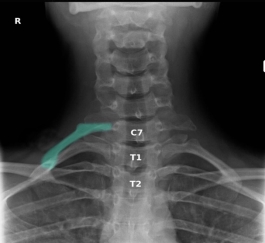
Fig. 3. Cervical rib X-ray cervical spine/upper chest (AP view) A prominent cervical rib arises from the costal element of the C7 transverse process on the right side (green overlay). A very small cervical rib is also present on the left. The downward orientation of the C7 transverse processes compared to the upward orientation of the thoracic spine transverse processes helps differentiate the cervical ribs from rudimentary first ribs
Epidemiology
Primary effort thrombosis appears to be a relatively uncommon condition. Based on data from Sweden, its incidence is estimated to be around 2.03 cases per 100,000 people annually. In our regional referral center, however, we have observed only 1.0 case per 100,000 population per year over the past five years. If the actual incidence falls between one and two cases per 100,000 people each year, this would translate to approximately 3,000 to 6,000 cases annually in the United States. Upper extremity effort thrombosis constitutes about 1 % to 4 % of all venous thrombosis episodes, which suggests a slightly higher estimate of its prevalence. Like many «rare» conditions, it is likely under-recognized by nonspecialists. The mean age at presentation is in the early thirties, with a male-to-female ratio of approximately 2:1. This condition is more frequently observed on the right side of the body, likely due to right-hand dominance; additionally, between 60 % and 80 % of patients report a history of vigorous upper extremity exercise or activity. A recent review involving one major league and one Division I baseball team over an eleven-year span identified four cases of primary effort thrombosis, resulting in an incidence rate of one case every five years for high-level baseball clubs. Determining the relative rate of primary effort thrombosis compared to secondary thrombosis is challenging due to a lack of comprehensive studies. However, two surveys conducted at different institutions indicate that primary effort thrombosis accounts for approximately 40 % of all upper extremity thrombosis episodes; it should be noted that both studies involved small sample sizes.
Pathogenesis
Effort thrombosis typically occurs after engaging in sports that require intense and sustained upper body movements, such as wrestling, ball games, gymnastics, and swimming. It is thought that the arm's retroversion, hyperabduction, and extension during these activities place excessive stress on the subclavian vein, resulting in microtrauma to the endothelium and triggering the coagulation cascade. There is significant evidence supporting the involvement of anatomical abnormalities at the thoracic outlet—such as cervical ribs, congenital bands, hypertrophied scalene tendons, and abnormal costoclavicular ligament insertions—in the development of effort thrombosis. (Figures 4 and 5 illustrate normal and abnormal thoracic outlet anatomy.) The constricted costoclavicular space causes vein compression and flow stasis while also limiting subclavian vein mobility, making it more vulnerable to injury from arm movements. This creates a self-reinforcing cycle of endothelial damage, thrombosis, and recanalization. Repeated endothelial injury leads to intimal hyperplasia, inflammation, and fibrosis, which contribute to venous webs, extensive collateral circulation formation, and perivenular fibrosis. Consequently, this exacerbates stasis and crowding in the costoclavicular area. Effort thrombosis has thus been appropriately classified as a venous variant of thoracic outlet syndrome.
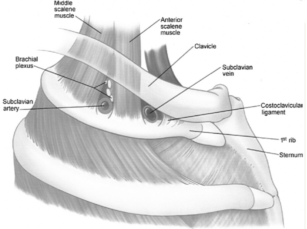
Fig. 4
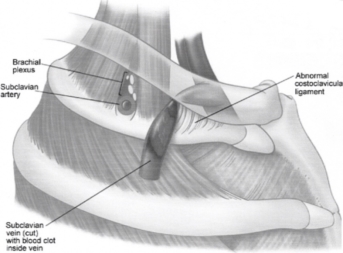
Fig. 5
Some researchers have noted a higher prevalence of factor V Leiden mutation, prothrombin gene mutation, and other inherited thrombophilic conditions among patients with idiopathic upper extremity deep vein thrombosis (UEDVT). A recent study by Cassada et al. found that about two-thirds of patients with Paget-Schroetter syndrome (PSS) also had concurrent thrombophilia; they further indicated that this coexisting condition was linked to increased postoperative complications following corrective surgery. However, other studies have challenged this association by showing that the prevalence of inherited thrombophilias in individuals with effort thromboses is similar to that in the general population. They observed that elevated rates of concurrent thrombophilic disorders were confined to patients with idiopathic UEDVT unrelated to effort.
Thus far, unlike venous thrombi found in other areas (such as lower extremities or visceral veins), the influence of inherited or acquired thrombophilic disorders on the onset and progression of effort thrombosis remains ambiguous. In conclusion, anatomical abnormalities causing costoclavicular crowding along with repetitive endothelial trauma from muscular exertion are critical factors driving both the initiation and progression of effort thrombosis. Inflammation appears to play a supplementary role by contributing to perivenular fibrosis and adhesions that perpetuate obstruction. The impact of inherited or acquired thrombophilic disorders is still uncertain and warrants further research.
Clinical Presentation
The clinical manifestations of thoracic outlet syndrome (TOS) vary based on the specific anatomical structure that is compressed and tend to be more pronounced during and after activities involving overhead movements.
- Brachial Plexus Compression (95 % of cases):
– Sensory loss or paresthesia following the ulnar nerve distribution
– Pain in the neck and arm — Gilliatt-Sumner hand: atrophy of intrinsic hand muscles, including the thenar, hypothenar, lumbrical, and interosseous muscles
- Subclavian Vein Compression (up to 3 % of cases):
– Swelling
– Venous distention
– Diffuse pain in the hand or arm
– Heaviness in the affected area
– Increased risk of arm thrombosis (Paget-Schroetter disease)
– Subclavian Artery Compression (<1 % of cases):
– Mild ache and fatigue in the arm — Symptoms known as the "5 Ps»: pulselessness, pain, pallor, paresthesia, and poikilothermia
– A decrease in blood pressure greater than 20 mm Hg in the affected arm compared to the opposite arm — Ischemia may result in ulcerations and gangrene in the affected limb.
Diagnosis
Patients commonly present within 24 hours of an inciting event, with history of excessive activity of the upper extremity and/or dehydration. The upper extremity and chest is painful, congested, and cyanotic appearing. Superficial veins can appear engorged and occasionally thrombosed veins can be palpated in the axilla. Pain involving the affected arm is characteristically sudden, involving the dominant extremity. Physical exam provocative tests have a high false positive rate and are more likely to detect neurogenic Thorasic outlate syndrom rather than Venous TOS.
Paget-Schroett Syndrom can also present with or be complicated by pulmonary embolism (PE). Studies report rates of PE and PSS ranging from 20–30 %. Among upper extremity deep vein thromboses (DVT), PE occurs more often with secondary upper extremity DVT. When considering all DVT, PE is more associated with lower extremity DVT than upper extremity DVT. Although the risk of PE is smaller in PSS than other DVT states, it is important to be aware of the phenomenon.
Diagnosis is usually achieved with history and physical examination, and can be confirmed with imaging. Initial evaluation includes duplex ultrasonography revealing partial or complete thrombosis of the axillary and/or subclavian veins.
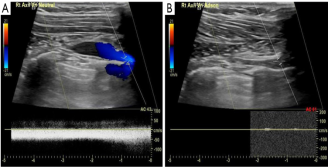
Fig. 6. Positive confirmatory ultrasound with provocative occlusion of the axillosubclavian vein. (A) Ultrasound demonstrating flow within the right axillosubclavian vein with arm in neutral position; (B) occlusion of right axillosubclavian flow after provocat
Duplex imaging is the gold standard in diagnosis with reports of 80–100 % sensitivity and specificity
If duplex ultrasound is inconclusive, other imaging techniques may be used to confirm the diagnosis. Catheter-directed venography was historically the gold-standard for diagnostic imaging, but due to its invasive nature, higher costs, and accuracy of non-invasive ultrasound, it is now reserved for cases with high clinical suspicion and equivocal non-invasive images. Venography via computed tomographic (CT) or magnetic resonance (MR) can also be performed in the setting of atypical symptoms or equivocal ultrasound to interrogate the surrounding anatomy. However, both have their drawbacks.
CT venography is complicated by risk of contrast and radiation exposure, while MR carries high costs and limited availability. When faced with an inconclusive non-invasive ultrasound and high clinical suspicion for upper extremity DVT, providers have the option of choosing among the above venography techniques. Alla et al. propose an algorithm to follow equivocal Doppler ultrasound with MR venogram if a high index of suspicion and no alternative explanation exists. A positive MR venogram is then followed by catheter-directed thrombolysis and early thoracic outlet decompression in patients with symptoms present for less than 2–6 weeks
Plasma D-dimer levels may be elevated with upper extremity DVT, however specificity ranges from 14–60 %. As Paget-Schroett Syndrom is a rare disorder, no guidelines exist on routine D-dimer testing. It may be useful as an adjunct, but is not recommended as a confirmatory test.
Patients complaining of neuropathic symptoms warrant nerve conduction studies, although the venous swelling can sometimes produce paresthesias unrelated to neurogenic thorasic outlate syndrom. Routine hypercoagulable workup for upper extremity thrombosis is not recommended. However, if a patient presents with an unexplained thrombosis and/or family history, hypercoagulable work-up should be performed. Hypercoagulable investigations include mutations in factor V Leiden and prothrombin G20210A, and deficiencies in antithrombin, protein C, and protein S. Lupus anticoagulant screening and anticardiolipin/anti-β2-glycoprotein antibodies may also help direct clinical management.
Cancer screening following unexplained upper extremity thromboses follows similar recommendations for those in the lower extremities. Routine screening is not recommended, however may be considered in unexplained thromboses. In these cases, a thorough history and physical examination, routine laboratory studies, abdominal ultrasound, chest X-ray as well as any other age or gender specific screenings should be performed.
Treatment
The management of PSS focuses on alleviating symptoms caused by obstruction, preventing complications from deep vein thrombosis (DVT), and reducing the risk of recurrence. This can be accomplished through anticoagulation, thrombolysis, and/or surgical decompression. The first step in treatment is to initiate systemic anticoagulation immediately after diagnosis. While not specifically tailored for PSS, the 2016 CHEST Guideline and Expert Panel Report on antithrombotic therapy for venous thromboembolism (VTE) recommends using dabigatran, rivaroxaban, apixaban, or edoxaban for patients with VTE who do not have cancer, favoring these over vitamin K antagonists. In contrast, vitamin K antagonists are preferred over low-molecular-weight heparin.
Generally, anticoagulation alone is not recommended for treating PSS. A more aggressive strategy that includes thrombolysis and surgery has been shown to yield better patient-reported outcomes, such as symptom resolution and return to work. If there are no contraindications, it is optimal to administer therapeutic anticoagulation for at least five days before performing venography and catheter-directed thrombolysis within two weeks of symptom onset. Early catheter-directed thrombolysis has a success rate reported between 75 % and 84 %. However, treating clots older than two weeks is less effective since the thrombus becomes chronic and less responsive to thrombolytic therapy; one study indicated a success rate of only 29 % for thrombolysis performed 2–12 weeks after symptoms began.
Despite initial symptom relief from anticoagulation and thrombolysis, re-thrombosis can occur in up to one-third of patients. Therefore, more definitive treatment through thoracic outlet decompression is advised for those who are suitable surgical candidates. In cases without specific anatomical abnormalities, decompression typically involves first rib resection via transaxillary, supraclavicular, or infraclavicular approaches; however, no clinical trials have established the superiority of any particular method. If a cervical rib is present, it should also be excised. Additionally, if necessary, partial resection of the subclavius muscle and anterior scalene muscle may be performed to reduce thoracic outlet bulk and help prevent symptom recurrence.
There is currently no consensus on the duration of anticoagulation for post-thrombotic syndrome (PSS). The 2016 CHEST Guideline and Expert Panel Report on antithrombotic therapy for venous thromboembolism (VTE) recommends a three-month course of treatment following any upper extremity deep vein thrombosis (DVT), irrespective of thrombolytic interventions. Some experts advocate for a more individualized approach that incorporates postoperative venography. If vein patency is confirmed, further intervention is unnecessary, and anticoagulation can be discontinued. Conversely, if persistent stenosis or re-thrombosis is detected, anticoagulation should continue, with monthly duplex ultrasound examinations performed for six months. While additional procedures such as surgical thrombectomy, balloon venoplasty, and stenting have been previously employed for persistent stenosis or re-thrombosis, they are now generally discouraged due to low success rates and high morbidity.
Surgical procedures
The standard procedure, previously detailed, involved an anterior subclavicular partial resection of the anterior half of the first rib, removal of the subclavius tendon, division of the anterior scalene tendon, and resection of part of the middle scalene muscle up to the level of the subclavian artery. Additionally, a vein patch plasty was performed on the stenotic segment of the subclavian vein using a short segment of saphenous vein harvested from the upper thigh to achieve a larger diameter. The operation was conducted extrapleurally.
During the removal of the first rib, subclavius muscle, and scalene muscles, no anticoagulation was administered. Once the vein was fully isolated and prepared for clamping and opening, an intravenous bolus of heparin (100 U/kg) was given. Simultaneously, to prevent platelet aggregation, a bolus of low-molecular-weight dextran (40,000 Da) in saline solution (50 mL) was administered by anesthesia; this dextran infusion continued at a rate of 15 mL/h without interruption.
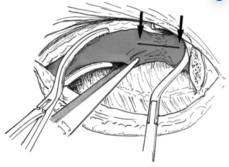
Fig. 7
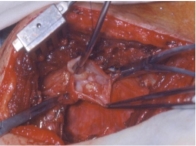
Fig. 8
After opening the vein, all fibrous tissue and organized thrombus were excised (Fig 7). The vein patch was placed over the venotomy site (Fig 8), clamps were released, and the procedure concluded. An extrapleural drain (Jackson-Pratt round drain from Cardinal Health) was left in place for 48 to 72 hours until drainage fell to ≤30 mL per 24-hour period. Antibiotics were administered only during surgery and for 48 hours postoperatively until removal of the extrapleural drain.
Complications
Paget-Schretter syndrome is associated with several complications, including recurrent thrombosis, pulmonary embolism, and post-thrombotic disease. While some studies indicate a lower incidence of pulmonary embolism compared to phlebothrombosis in the lower extremities and catheter-related issues, the risk of serious complications remains significant. The prevalence of post-thrombotic disease in the primary condition can be as high as 46 %. Additionally, venous gangrene may occur very rarely during the acute phase.
Prognosis
Following PSS treatment can lead to significant morbidity from post-thrombotic syndrome (PTS), which manifests as pain, heaviness, and swelling in the affected limb and can become a chronic and debilitating condition. PTS affects 7–46 % of patients with upper extremity DVT, occurring more frequently in primary than secondary cases. Although it is challenging to prevent all instances of PTS, evidence suggests that early intervention correlates with better patient-reported outcomes. Success rates of 90–95 % have been observed when PSS is diagnosed and treated promptly with immediate decompression. Urschel et al. reported that 95 % of patients experienced “excellent/good” results regarding pain relief, employment status, and recreational activities following early thrombolysis and first rib resection. Other studies have echoed these findings, reinforcing the benefits of early surgical decompression. Patients with venous thoracic outlet syndrome (VTOS) who undergo first rib resection and scalenectomy report significant improvements in quality of life, often achieving near-complete recovery within one year post-surgery. Recurrence rates are low; only 18 % of postoperative VTOS patients require physical therapy to alleviate symptoms without necessitating further surgical interventions. A recent meta-analysis on surgical outcomes for thoracic outlet syndrome indicated that surgical intervention is both safe and effective, with 90 % of VTOS patients reporting “excellent/good” outcomes; however, the variability among studies complicates drawing definitive conclusion
Conclusions
Rapid diagnosis and treatment for PSS is essential for good outcomes. Treatment involves immediate anticoagulation. Venography with catheter-directed thrombolysis can confirm the diagnosis and is potentially therapeutic. Surgical decompression should follow soon after, both for completion of treatment and prevention of recurrent thromboses. Managed in this fashion, the vast majority of patients with VTOS report beneficial outcomes with near full recovery at 1 year.
References:
- Farrar TA, Rankin G, Chatfield M. Venous thoracic outlet syndrome: approach to diagnosis and treatment with focus on affected athletes. Curr Sports Med Rep 2014;13:81–5. [Crossref] [PubMed]
- Kraaijpoel N, van Es N, Porreca E, et al. The diagnostic management of upper extremity deep vein thrombosis: A review of the literature. Thromb Res 2017;156:54–9. [Crossref] [PubMed]
- Fliegel BE, Menezes RG. Anatomy, Thorax, Cervical Rib. StatPearls. 2020. pmid: 31082045.
- Nichols AW. Diagnosis and management of thoracic outlet syndrome. Curr Sports Med Rep.; 8(5): pp. 240–9. doi:
- C7ribMark» by James Heilman, MD, Wikimedia Commons, licensed under CC BY-SA 4.0.
- Hangge P, Rotellini-Coltvet L, Deipolyi AR, Albadawi H, Oklu R. Paget-Schroetter syndrome: treatment of venous thrombosis and outcomes. Cardiovasc Diagn Ther. 2017 Dec;7(Suppl 3):S285-S290. doi: 10.21037/cdt.2017.08.15. PMID: 29399532; PMCID: PMC5778512.
- Fugate MW, Rotellini-Coltvet L, Freischlag JA. Current management of thoracic outlet syndrome. Curr Treat Options Cardiovasc Med 2009;11:176–83. 10.1007/s11936–009–0018–4
- Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010 Sep;11(4):358–62. PMID: 21079709; PMCID: PMC2967689.
- Dep A, Concannon E, Mc Hugh SM, Burke P. Paget-Schrotter syndrome and complications of management. BMJ Case Rep. 2013 Jul 12;2013:bcr2013008858. doi: 10.1136/bcr-2013–008858. PMID: 23853011; PMCID: PMC3736202.
- Farrar TA, Rankin G, Chatfield M. Venous thoracic outlet syndrome: approach to diagnosis and treatment with focus on affected athletes. Curr Sports Med Rep 2014;13:81–5. [Crossref] [PubMed]

