A critical analysis of the biological exploitation, and mechanisms of resistance to this review to highlight the importance of Apocynum cultivation as an ideal option for vegetation restoration on saline soils in the arid region of the Aral Sea. In the saline areas of the Aral Sea, where the cultivation of crops is almost impossible due to the high salt toxicity of the soil, the cultivation of Apocynum can reduce salt levels in the soil and provide a livelihood in the form of high-quality fiber. The review of the literature has shown that general salinity tolerance in Apocynum is related to the regulation of redox and ion homeostasis, but their molecular regulation has not yet been identified. The results obtained, such as sodium (Na+) extraction from soil and increased accumulation of flavonoids and antioxidants in leaves under salt stress, provide useful insights into the complex network of salt tolerance in Apocynum.
Apocynum belongs to the family Apocynaceae, identified as a genus of plants with high-stress tolerance. Apocynum Venetum and Pictum can be used as medicinal plants for the prevention and treatment of hypertension, the stem of the plant is used for textile production and is a source of good and quality fiber. Apocynum is known as '' in Russian literature, in China, the two species Apocynum Venetum and Apocynum Pictum are locally used as Luobuma, and these two species are utilized as traditional herbs and commodity crops and are also used for the phytoremediation of degraded saline land.
The Aral Sea is located in the northern desert part of Central Asia within Uzbekistan and Kazakhstan. The Aral Sea basin includes two major river basins, the Amu Darya and the Syr Darya. The Amu Darya and the Syr Darya flow through the territories of six countries: Kyrgyzstan, Tajikistan, Uzbekistan, Kazakhstan, Turkmenistan, and Afghanistan [1]. Not so long ago, the Aral Sea was the fourth largest lake in the world, renowned for its rich natural reserves and the Aral Sea area was considered a thriving and biologically rich natural environment. The unique enclosure and diversity of the Aral Sea left no one indifferent.
The decision made in 1965–1990 by the centralized authorities of the country (USSR) without considering ecological requirements on the increase of cotton and other crops production by means of the rapid growth of irrigated lands in the region predetermined the future death of Aral Sea because total flow to the Aral Sea decreased from 55,0 km3 in 1961 to 8–10 km3 by 1990 [1]. The native riparian vegetation suffered under those circumstances, and irrigated agriculture, particularly along the lower reaches, experienced water shortages that resulted in ecological degradation and financial losses, respectively. Soil salinization has grown to be a serious worry along with the expansion of irrigated areas and times of water shortage [3]. The salinization of the soil has become a significant problem for local farmers along with the expansion of irrigation areas and periods of water scarcity [4].
In addition, the adoption and practical implementation of various sexual and asexual propagation techniques also represent important factors in the successful cultivation of Apocynum in salt-affected soils. In summary, there are great prospects for actively developing the Apocynum industry by utilizing the saline-alkaline lands of the Aral Sea and conducting research on Apocynum cultivation technology and conservation strategies in arid lands, which we explain in the future.
- Taxonomy and geographical distribution of Apocynum in Central Asia: Apocynum, its distribution and botanical description
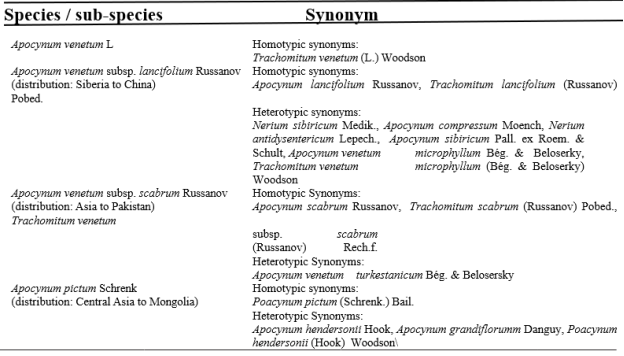
Nine species belong to the genus Apocynum, which is found in all climatic regions of North America, Europe, and Asia. The Apocynum is a member of the class Dicotyledonae, order Gentiales, and family Apocynaceae. Just the two Apocynum species A. venetum and A. pictum currently found in Central Asia. Both the A. venetum and the A. pictum species are referred to as Apocynum in Central Asia [5]. Synonymy details for members of two Apocynum species and two subspecies of A. venetum are described in Table 1. 1 for Central Asia.
The arid and semi-arid regions of Central Asia are home to two perennial semi-shrub species, Apocynum venetum, and A. pictum. Both species are found in the riparian vegetation that grows beside rivers and streams in the Central Asian drylands, as well as on alluvial plains, the edges of deserts, and the courses of permanent and episodic rivers [6;7:8;]. Kendyr is most common in the Central Asian republics, especially in the floodplains of the Syr Darya, Amu Darya, Panj, Vakhsh, Ili, Chu, Talas, Black Irtysh, Lepsa, Ak-Su rivers and on the banks of Balkhash, Zaisan and Akulskoye lakes. In Central Asia, the Apocynum is found mainly in Kazakhstan. In most cases, the Apocynum grows in continuous patches among other vegetation. The following types of Apocynum are found in Central Asia: Lancet — found in the valleys of the Central Asian rivers Syr Darya, Chu, and Ili; salt tolerant — growing only in the Ili region; rough — common in the Amu Darya valley. The Apocynum with the highest fiber quality, Apocynum lanceolate and coarse, grows only in Central Asia [9;10]. Kendyr (Кендыр) is applied in Russian literature as a common name for Apocynum. The Kazakhs often call this plant “kzyl ”, which means red, as the shoots often have an easily recognizable red coloration. Kendir, which grows in riparian forests, is called «grey kendir» by the Kazakhs. Other synonyms for kendir are also known, such as «turka» or «torka», but these refer to fiber.
Table 1
Subspecies and synonyms of A. venetum and Apocynum pictum including their spread [5]
- Morphological review of Kendir
The Kendir is a perennial plant with root shoots. The above-ground part of the Kendir — the stems — dies off every autumn and grows back in the spring, while the underground part — the root system — grows for several years. The appearance of the plant varies noticeably depending on the growing conditions. In open areas, meadows, and plantations the shrub consists of 5–10 stems, which are not very tall and strongly branched. In shady areas, dense reeds, or forests, the number of stems per bush is much lower. The stems grow very high, sometimes reaching a height of 4–5 meters, and are not very branched [6;9]. The Apocynum stem is cylindrical, slender, smooth, and flexible, with a great variety of colors. Branches, thinner than the stem, alternate or oppose and branch off the stem to the sides and upwards, usually at an angle of 45 to 60 degrees. Plants from the Amu Darya region have a predominantly opposite leaf arrangement, the Chu River has an alternating leaf arrangement and the Syr Darya has a mixed leaf arrangement [9].
The underground part of an adult plant consists of four main parts with different functions: the vertical rhizome (underground stem), the vertical roots, the horizontal roots for propagation, and the tubercles of the feeding systems. The vertical rhizome is an extension of the above-ground shoot. It is essentially a perennial part of the stem. Vertical roots enable the plant to survive when groundwater is relatively deep. Horizontal roots are the underground reproductive organs of the Apocynum. The dormant buds are hidden in the folds of the bark. Under favorable conditions or when the roots separate from the mother plant, the individual buds awaken and grow into vertical roots and above-ground shoots. Due to the large number of nutrients stored in the dormant buds, the rhizome produces new shoots each year. This biological feature is used for vegetative propagation.
-
The benefits of planting Apocynum
- Source of fiber
The Apocynum plant is a significant agricultural crop, both medically, ecologically, and economically (Figure 1). Apocynum belongs to the fiber crop and its fiber is famous as the “king of the wild fibers” [11]. A. venetum was first used for the production of fiber in 1930 at the Union of Soviet Socialist Republics (USSR). After the last world war, there was a call for creating large fields for the use of A. venetum as a textile plant [6]. Apocynum fibers are comparable to bast fibers; they resemble those of hemp (Cannabis sativa L.) as well as flax (Linum usitatissimum L.), although Apocynum fiber bundles (30–40 fibers) tend to be more resistant than flax (L. usitatissimum) (10–30 fibers). Tough bast fibers derived directly from the inner part of the bark, are utilized for making clothes, ropes, sails, fishing nests, and quality paper. In addition to bast fibers, A. venetum produces thread fibers from the fruit [12;13]. Apocynum fiber, on account of its antimicrobial and anti-UV properties, is considered a natural functional product. The degree of inhibition of Apocynum fiber compared to cotton fiber (Gossypium hirsutum L.) for Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans is 47.7 %, 69.0 %, 56.6, % and 40.1 % respectively [13]. In comparison with cotton, Apocynum textiles retain warmer temperatures, offer higher aeration, and absorb more UV light [18]. However, there are significant differences between Apocynum fibers and cotton. The modulus of elasticity of all Apocynum fibers from the three treatments exceeds 300 cN/dtex, while the modulus of elasticity of cotton is only 96 cN/dtex, meaning that textiles made from Apocynum can be less soft than those made from cotton. In addition, the tear work of the Apocynum fibers with 11.82–13.72 µJ/dtex, is less than the respective value of 16.77 µJ/dtex for cotton. Thus, Apocynum fibers may be less strong than cotton fibers [19]. Bacterial cell walls are destroyed on exposure to Apocynum fiber [14]. Apocynum stem cells contained tannins, making the fibers tolerant to microbial decomposition [10]. Increasing attention is now focused on the isolation, purification, and study of the mechanism of action of antimicrobial and anti-ultraviolet (UV) bioactive compounds within Apocynum fibers [16;17].
The chemical and physical properties of Apocynum fibers are determined by their basic composition [20]. As with hemp (C. sativa) or flax (L. usitatissimum) fibers, Apocynum fibers contain cellulose, hemicellulose, lignin, pectin, water-soluble substances, wax, ash, and others. The amount of pectin in Apocynum is 13.14 %, and the proportion of water-soluble substances is 17.22 %, which is the highest of all hemp fibers. It has a lignin component of 12.14 %, which is higher than that of ramie, flax, abaca, and sisal. Compared with flax fiber, the cellulose content ranges from 40.82 %, the lowest of all hemp fibers, to 72 % [11]. For the pectin to be extracted, the Apocynum fiber must be degummed. A specific kind of polysaccharide is hemicellulose. The fiber's spinnability increases with decreasing hemicellulose content. The extraction of Apocynum fiber is hampered by the high lignin concentration of the plant's phloem. The extraction of Apocynum fibers is highly challenging due to its unique phloem tissue [21].
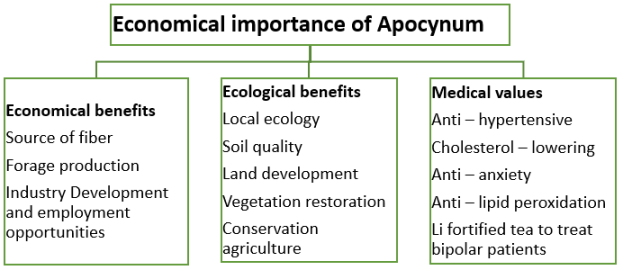
Fig. 1. Economic importance of Apocynum in our daily life. The cultivation of Apocynum comes with several economical, ecological, and medicinal values
3.2 Therapeutic Properties of Apocynum Fibers
Apocynum's pharmacology and phytochemistry have both been well investigated [22]. Due to its antihypertensive [23], cholesterol-lowering [24], and anti-anxiety [25] properties as well as its action against lipid peroxidation [26] and oxidation of low-density lipoproteins [24], Apocynum leaf extract is effective for the treatment of many diseases. In addition, it has positive effects on atherosclerosis and ischemic reperfusion myocardial damage [27;28]. Besides this, Apocynum leaf tea is considered a health drink in China, Japan, and other East Asian countries, making it even more available in North America in the last few years [29]. According to modern scientific research, Apocynum's broad pharmacological activity is linked to the availability of a variety of flavonoids [22]. Hyperoside acts against corticosterone-induced neurotoxicity and atherosclerosis; kaempferol has anxiolytic properties; and quercetin suppresses tumor development, destroys free radicals, and lowers blood pressure [30; 31]. Lithium (Li) is used in the treatment of bipolar periodic and monopolar periodic manic-depressive disorder and has some effect on periodic schizotypal psychosis, pathological impulsive aggression, premenstrual tension, alcoholism, and drug abuse [32;33]. Use of lithium is non-psychiatric use and has been used to treat physical activity disorders, thyroid disorders, abnormal secretion of antidiuretic hormone, granulocytosis, seborrhoeic dermatitis, herpes, etc. Lithium nutritional supplementation can control mood, and decrease criminality, suicidality, drug addiction, etc [34]. Plants of Apocynum in Xinjiang include fairly high Li content with the potential to be used as a health tea, and as a supplementary source of deficient dietary micronutrients [35].
- Apocynum's mechanisms and response to salinization
4.1 Apocynum Growth in response to Salinity and protective tolerance mechanisms in Apocynum
Salinity may affect Apocynum at four distinct growth stages, according to the investigations Seeds that are young and during the germination period can tolerate salt. [36]. In A. venetum, low NaCl concentrations (50 mmol L 1) encourage seed germination while high NaCl concentrations (> 200 mmol L 1) decrease the proportion of germination. Apocynum growth is enhanced by low salinity, possibly as a result of the activation of early signaling pathways and defense mechanisms. Salt stress also inhibits a number of additional characteristics, including seedlings. height, root length, leaf length, and leaf width [37]. A. venentum has a fresh weight, but the 100 mmol L1 NaCl greatly reduces its not dry weight. Due to Apocynum's reduced photosynthetic rate and maximal photochemical effectiveness, growth is inhibited by high salinity and drought. In the Apocynum leaf tissues, salinity lowers membrane integrity, relative water content, and growth trends but raises the contents of lipid peroxidation, malondialdehyde (MDA), proline, and hydrogen peroxide (H2O2). Contrary accounts, however, indicate that this plant species can withstand up to 300 mmol L-1 of NaCl [38;39].
4.2 Apocynum's tolerance mechanisms and oxidative defense
Apocynum is a type of halophytic plant that has a sophisticated system for coping with abiotic stress. The adoption of Apocynum under saline conditions was the subject of this review and several others. Apocynum is a type of halophytic plant that has a sophisticated system for coping with abiotic stress. A number of biochemical and molecular mechanisms may be possibly involved in Apocynum's resistance to the saline ecosystem and its utility for the remediation of saline areas, as this review concentrated on the potential adoption of Apocynum under saline conditions. Nevertheless, the existing research has been critically examined and theorized as a salinity tolerance mechanism in Apocynum, which needs further validation. There is very little data accessible showing precise stress tolerance mechanisms in Apocynum.
The generation of reactive oxygen species (ROS) is a necessary physiological mechanism in plants (Shahzad et al., 2021), but under extreme circumstances, these ROS cause oxidative harm to nucleic acids, disrupt ionic homeostasis and impair photosynthetic capacity. Nevertheless, ROS at their normal level plays an important role in several key physiological mechanisms in plants. Because of its superior ROS scavenging system, the halophytic plant species Apocynum Venetum exhibits extremely high amounts of stress resistance. In the salty environment of Apocynum, a number of important antioxidants have various functions in the scavenging of ROS (Fig. 2). In Apocynum salt-treated, higher levels of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbic acid activity are seen. (Chen et al., 2018). Apocynum also employs multiple strategies to combat salt stress, including the stimulation of peroxidase enzymes for ROS scavenging, according to Chen et al. (2020). The Medicago sativa plant's AvDH1 homolog, MH-1, increases in expression after exposure to NaCl or H2O2, and transforming it in Arabidopsis results in the development of the two salt- and ROS-tolerant forms. By increasing the ability for oxidative collecting, an analogous function of the DEAD-box family suggests a possible role for the DEAD-box helicase genetic family in salt tolerance in Apocynum. Oxidative digestion is a crucial mechanism in Apoycynum, but it can be activated in different ways depending on stress, tissue specificity, development stage, and cultivation. Increasing or maintaining Apocynum's ability to tolerate salinity may be possible by focusing on the ROS scavenging mechanism to enhance ROS detoxification.
4.3 Osmosis and ionic homeostasis regulation
A shortage of water in cells under salinity-related circumstances is a typical physiological constraint that drastically reduces production. In response, the plant created a variety of osmoprotectants and osmolytes. These substances shield membranes and proteins from the reducing effects of abiotic stress settings. They are tiny molecules with an unaltered electric charge and are non-toxic at molar concentrations.
Little is known about the physiological and molecular control of osmotic adjustment and its function in adaptation to salinity, especially with the example of Apocynum. Nevertheless, a causal relationship has been established and the research that is currently available has been examined to comprehend the role of osmolytes in the setting of adaptation to salinity in Apocynum. The buildup of soluble sugar and soluble protein increases significantly under osmotic stress, increasing the salinity tolerance of A. cannabinum and A. venetum. In a comparable manner, greater osmotic adaptation (higher amino acid proline and dissolved sugar level) and a better ROS scavenging system (higher SOD, APX, and glutathione reductase (GR) activity) are strongly correlated with A. venetum's ability to withstand oxidative stress. Proline is an important stress regulator in plants, with an increase in Pro levels under salt stress. P5CS is important for producing proline and protecting cells from osmotic stress.
An additional group of biomolecules with Osmo- and antioxidant characteristics are flavonoids. In Apocynum, stress circumstances have a significant impact on flavonoid biosynthesis, with salt stress inhibiting the expression of chalcone synthase, chalcone isomerase, and flavonoid 3-O-glucosyltransferase. De novo assembly of 105.61 Gb of sequence data and 715 million high-quality reads reveals that 2822 differentially expressed genes (DEGs) are enriched in processes linked to flavonoid metabolism. The amounts of quercitrin, hyperoside, and total anthocyanin are significantly higher than those in A. hendersonii, indicating the potential role of flavonoids in enhancing over Li tolerance. A combined and comparative metabolome and transcriptome analysis is done in A. venetum to elucidate the flavonoid biosynthesis pathways.
For life and stress tolerance, ionic homeostasis control is crucial. Na+ toxicity in Apocynum is brought on by raised Na+ absorption and the generation of ROS. Na+ vacuolar sequestration via Na+/H+ transport (NHX) and ROS detoxification via the antioxidant defense mechanism are crucial for preventing or reducing K+ shortage. According to the latest research, K+ and Na+ concurrently play essential roles in osmotic adjustment, the maintenance of water status, and the preservation of transpiration, all of which are necessary for A. venetum to deal with stress caused by drought.
Strong potassium (K+) assimilation is a characteristic of Apocynum venetum, with tissue K+ concentrations remaining constant after 5 weeks of low K+ therapy. AKT1, a component of the Shaker-like K+ channel family, was cloned, and conveying an analysis of it revealed that it is highly activated and increases K+ uptake in Apocynum stems in reaction to salt distress. In addition to halophytic plant species, including Suaeda salsa and Puccinellia tenuiflora (Grisebach) Scribner & Merrill, have also been linked to this function of AKT1 in controlling K+ absorption. Plans for restoring and photo-remediating salty soils could be obtained by focusing on and examining AKT1.
Conclusion
According to the literature study, A. venetum and A. pictum produce textile-grade fibers, but they work best when combined with cotton or other natural or synthetic fibers. Due to the porous composition of the Apocynum fibers, these yarns have excellent oxygenation and heat qualities and are primarily used to make underwear.
Apocynum leaves are also used to make anti-hypertonic tea and medicine, according to the books. Apocynum species produce fibers and leave over sizable areas in Central Asia's arid environment without watering as they use groundwater. Additionally, compared to cotton, Apocynum species can tolerate greater soil salinity levels. More saline and arid circumstances can be tolerated by A. pictum than A. venetum. In circumstances that are unfavorable for producing crops with irrigation, Apocynum species are able to be utilized and earn a living for the local population. These circumstances include saline soils and/or an unreliable water source for agricultural systems.
References:
- UNECE, 2010. Problems of the Aral Sea Basin and the Importance of Regional Cooperation of regional cooperation [online]. Available at: https://unece.org/fileadmin/DAM/SPECA/documents/ecf/2010/AralSea.pdf
- Treshkin, S.Y, 2001. The Tugai Forests of floodplain of the Amudarya River: Ecology, dynamics and their conservation. In: Breckle, S.W., Veste, M., Wucherer, W. (eds.), Sustainable land use in deserts, 95–102. Springer, Heidelberg.
- Kuzmina, Z.V., Treshkin, S.Y., 1997: Soil salinization and dynamics of Tugai vegetation in the southwestern Caspian Sea region and in the Aral Sea coastal region. Eurasian Soil Sci. 30, 642–649.
- Royal Botanic Gardens, 2012: URL http://apps.kew.org/wcsp (accessed on 02. February 2012)
- Pavlov, N.V., 1942: Dikie poleznye i tehnicheskie rastenija SSSR. Kazahskij Gozudarstbennyi Universitet im. S. M. Kirova and Kazahskij Filial Akademii, Nauk, Moscow.
- Zhang, L., Li, H.Q., Yi, H.Y., 2003. Botanical characteristics, distribution and application of Lopnur Kender resources in Aksu prefecture. Xinjiang Agr. Sci. 40, 172–174.
- Zhang, G.L., Qian, X.S., Gu, G.P., 2005. Apocynum venetum L. Resources in Shanxi, Hubei, Henan and Shandong provinces and its cultivation techniques. Chin. Wild Plant Resour. 25 (6), 26–28.
- Berljand, S.S., 1950: Agro-Technology of. NAUK, Moscow
- Romanovich, V.V., Sharii, N.J., Zubzova, Z.D., Kazenas, L.D., 1951: Kendir v Kasahstanie. Kazah. Goz. Ezd., Almaty
- Li, H., Li, Y., 2006. The king of wild fiber — Apocynum verteura l. Shandong Fangzhi Jingji 134, 80–81.
- He, R.Y., Chu, J.P., Chen, G.L., 1997. China’s fiber crops. Chinas Fiber Crops 19, 21–23
- Zhang, W.M., Xiao, Z.C., Zhang, G.L., Gu, G.P., 2006b. Study on ecotype of Apocynum in Xinjiang and its fiber quality. Chin. Wild Plant Resour. 25, 33–37.
- Zheng, L., Du, B., Xue, W., 2011. Screening and identification of Acinetobacter junii for Apocynum vernetum L. fiber enzymatic retting. J. Text. Inst. Proc. Abst. 102 (8), 675–680. https://doi.org/10.1080/00405000.2010.514726.
- Lü, R., Su, D.M., Meng, L., Zhang, L.S., 2006. Antibiotic properties of Apocynum venetum. Acta Acad. Med. Qingdao Univ. 42, 71–72.
- Li, C.H., Liu, S.Y., Song, Y., Nie, K., Ben, H.X., Zhang, Y.M., Han, G.T., Jiang, W., 2020. A facile and eco-friendly method to extract Apocynum venetum fibers using microwave-assisted ultrasonic degumming. Ind. Crop. Prod. 151, 112443 https:// doi.org/10.1016/j.indcrop.2020.112443.
- Xu, X.X., Gong, J.X., Zhang, T., Li, Z., Zhang, J.F., Wang, L., Huang, J.F., 2020a. Insights into antibacterial mechanism of Apocynum Venetum L. fiber: evolution of bioactive natural substances in bast during chemical degumming process. Ind. Crop. Prod. 151, 112419 https://doi.org/10.1016/j.indcrop.2020.112419.
- WEI, C., 2004: Growth, physical and chemical properties, and utilization of Apocynum fibers. Pinzhi Xingneng Fenxi 9, 25–26.
- WANG, L., HAN, G., ZHANG, Y., 2007: Comparative study of composition, structure, and properties of Apocynum venetum fi bres under different pretreatments. Carbonhyd Polym. 69, 391–397.
- Thevs, N., Zerbe, S., Kyosev, Y., Rozi, A., Tang, B., Abdusalih, N., Novitskiy, Z., 2012. Apocynum venetum L. and Apocynum pictum Schrenk (Apocynaceae) as multi-functional and multi-service plant species in Central Asia: a review on biology, ecology, and utilization. J. Appl. Bot. Food Qual. 85 (2), 159–167. https://doi.org/ 10.4314/ijbcs.v4i3.60517.
- Tan, Y.L., Xu, Y., Zhu, J., 2021. Study on properties and status of Apocynum venetum. Light Text. Ind. Tech. 50 (1), 16–18. https://doi.org/10.3969/j.issn.2095- 0101.2021.01.007
- Xie, W., Zhang, X.Y., Wang, T., Hu, J.J., 2012. Botany, traditional uses, phytochemistry and pharmacology ofApocynum venetum L. (Luobuma): a review. J. Ethnopharmacol. 141 (1), 1–8. https://doi.org/10.1016/j.jep.2012.02.003.
- Kim, D.W., Yokozawa, T., Hattori, M., Kadota, S., Namba, T., 2000. Effects of aqueous extracts of Apocynum venetum leaves on spontaneously hypertensive, renalhypertensive and NaCl-fed-hypertensive rats. J. Ethnol. Pharmacol. 72 (1–2), 53–59. https://doi.org/10.1016/S0378–8741(00)00197–5.
- Kim, D.W., Yokozawa, T., Hattori, M., Kadota, S., Namba, T., 1998. Effects of aqueous extracts of Apocynum venetum leaves on hypercholesterolaemic rats. Phytother. Res. 12 (1), 46–48. https://doi.org/10.1002/(SICI)1099–1573(19980201)12:13.0.CO;2-I.
- Grudmann, O., Nakajima, J.I., Seo, S., Butterweck, V., 2007. Anti-anxiety effects of Apocynum venetum L. in the elevated plus maze test. J. Ethnopharmacol. 110 (3), 406–411. https://doi.org/10.1016/j.jep.2006.09.035.
- Yokozaw, T., Kashiwada, Y., Hattori, M., Chung, H.Y., 2002. Study on the components of Luobuma with peroxynitrite-scavenging activity. Biol. Pharm. Bull. 25 (6), 748–752. https://doi.org/10.1248/bpb.25.748.
- Wang, P., Guo, Q., Wang, Q., Zhou, X.R., Wang, S.M., 2015a. PtAKT1 maintains selective absorption capacity for K+ over Na+ in halophyte Puccinellia tenuiflora under salt stress. Acta Physiol. Plant. 37 (5), 100. https://doi.org/10.1007/s11738–015–1846- 3.
- Wang, W., Liang, X., Fu, D., Ru, T., Li, R., 2015b. Apocynum venetum leaf attenuates myocardial ischemia/reperfusion injury by inhibiting oxidative stress. Am. J. Chin. Med. 43 (1), 71–85. https://doi.org/10.1142/S0192415X15500056.
- Xiang, J., Lan, R., Tang, Y.P., Chen, Y.P., Cai, D.F., 2012. Apocynum venetum leaf extract attenuates disruption of the blood-brain barrier and upregulation of matrix metalloproteinase-9/-2 in a rat model of cerebral ischemia-reperfusion injury. Neurochem. Res. 37 (8), 1820–1828. https://doi.org/10.1007/s11064–012–0796-z.
- Zheng, M.Z., Liu, C.M., Pan, F.G., Shi, D.F., Ma, F.S., Zhang, Y.C., Zhang, Y.J., 2012. Protective effects of the flavonoid extract from Apocynum vernetum leaves against corticosterone-induced neurotoxicity in PC12. Cell. Mol. Neurobil. 31, 421–428. https://doi.org/10.1007/s10571–010–9635–4.
- Liang, T.G., Yue, W.Y., Li, Q.S., 2010. Comparison of the phenolic content and antioxidant activities of Apocynum venetum L. (Luo-Bu-Ma) and two of its alternative species. Int. J. Mol. Sci. 11 (11), 4452–4464. https://doi.org/10.3390/ ijms11114452.
- Schrauzer, G.N., 2002. Lithium: occurrence, dietary intakes, nutritional essentiality. J. Am. Coll. Nutr. 21 (1), 14–21. https://doi.org/10.1080/ 07315724.2002.10719188.
- Shahzad, B., Mughal, M.N., Tanveer, M., Gupta, D., Abbas, G., 2017. Is lithium biologically an important or toxic element to living organisms? An overview. Environ. Sci. Pollut. Res. 24 (1), 103–115.
- Klemfuss, H., Schirauzer, G.N., 1995. Effects of nutritional lithium deficiency on behavior in rats. Biol. Trace Elem. Res. 48 (2), 131–139. https://doi.org/10.1007/ BF02789187.
- Wang, L., Jiang, L., Zhao, Z.Y., Tian, C.Y., 2014. Lithium content of some teas and their infusions consumed in China. Food Sci. Biotech. 23 (1), 323–325. https://doi.org/ 10.1007/s10068–014–0045–0.
- Shi, Q.M., Deng, F.Y., Wu, M.Y., Chen, D.D., Yin, C.H., 2014. Study on salt tolerance of Apocynum venetum Linn. and Poacynum hendersonii (Hook.f.) Woodson at stages of seed germination and seedlings growth. North. Hortic. 12, 128–133.
- Zhang, X.L., 2007. Effect of salt stress on the germination of Apocyman venetun seed. China Seed Ind. (5), 48–50.
- Chen, C.H., Wang, C.C., Liu, Z.X., Liu, X.H., Zou, L.S., Shi, J.J., Chen, S.Y., Chen, J.L., Tan, M.X., 2018. Variations in physiology and multiple bioactive constituents under salt stress provide insight into the quality evaluation of Apocyni Veneti Folium. Int. J. Mol. Sci. 19 (10), 3042. https://doi.org/10.3390/ijms19103042.
- Chen, C.C., Wang, C.C., Liu, Z.X., Cai, Z.C., Hua, Y.J., Mei, Y.Q., Wei, L.F., Liu, X.H., 2020. iTRAQ-based proteomic technique provides insights into salt stress responsive proteins in Apocyni Veneti Folium (Apocynum venetum L.). Environ. Exp. Bot. 180, 104247 https://doi.org/10.1016/j.envexpbot.2020.104247.

