Pectin is not only used in the food industry to make colorful foods, but it is also widely used due to its ability to release heavy metals and radioactive nucleotides from the human body. The extraction of pectin from the corn cob is carried out with an acid hydrolysis method. Obtaining food pectin from food industry waste is cheaper and remains an urgent problem in the food industry. At present, a corn cobs, after removing the seeds, is at best used as animal feed, therefore, it is not subjected to industrial processing due to the absence of the latter. Pectin obtained from corn cobs has high gelling properties; high technological efficiency can be obtained due to its less use in the development of confectionery products.
Introduction . Over the next 15–20 years, scientists from Ukraine, Russia, Kyrgyzstan, Tajikistan and Uzbekistan carried out a number of scientific studies to develop technologies for obtaining pectin from plants and to study the physicochemical properties of this polysaccharide. Pectin is not only used in the food industry to make colorful foods, but it is also widely used due to its ability to release heavy metals and radioactive nucleotides from the human body. According to modern theory, pectin has a linear structure. Pectin is based on a chain consisting of D-galacturonic acid. The chain has a pyronose configuration with a 1,4-L glycosidic linkage.
It is known from the literature that the extraction of pectin from a corn cob is carried out with an acid hydrolysis method. Obtaining pectin in this way differs in that the processes carried out during production are complex, the cycle of technological processes is long. Recently, works have been published in foreign countries that show that the extraction of pectin is carried out by the enzymatic method on a large scale.
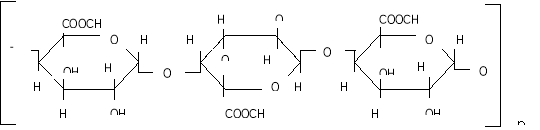
Methods and materials. The aim of our study was to use an enzymatic method to separate pectin from corn cobs. For this we used the Trichoderma harzinium enzyme. The corn cobs is crushed, passed through a sieve with holes with a diameter of 0.5 mm, 100 g of the finished powder is separated, sterilized in a thermostat at 110°C for 3 hours, 10 g of powder of the Trichoderma harzinium mushroom is added as an enzyme under aseptic conditions with bactericidal lamps. Boil 80 g of the prepared mixture, add distilled water cooled to 40°C and mix well. The finished mixture is stored for 10 days in a thermostat with a temperature of 36–38°C. Then the mixture is mixed with distilled water in a ratio of 1: 5 to extract the pectin which separated by the enzyme. The resulting extract is filtered using gauze, then centrifuged for 10 minutes in a centrifuge at 5000 rpm. The mixture is precipitated in 96 % ethyl alcohol in a 1: 2 ratio. The precipitate is cooled at + 8°C for 3 hours. Then the precipitate is separated with a filter, then washed with 80–96 % alcohol, dried in a thermostat at a temperature of 70–80°C, solid particles are ground to a powder state. The resulting pectin was 4 % of the bulk. The color of the pectin obtained is pale yellow, soluble in water. The purity is high, for example, the reaction to check for the presence of starch is negative, that is, the color does not change when a 1 % solution of a mixture of KI and I is added. The comparative viscosity of a 1 % aqueous solution of pectin is 1.06 cps. The molecular weight was determined by ultracentrifugation (50,000 rpm, 145 minutes, at 20°C) and was 26,600. To determine the amount of monosaccharides in pectin, 5 ml of 2n. sulfuric acid (H 2 SO 4 ) is added to 100 mg of pectin powder and hydrolyzed for 24 hours. The hydrolyzate is neutralized with barium carbonate salt (BaCO 3 ) and treated with KU-2. As a result of the treatment, the excess of carbonate ions is neutralized during hydrolysis. The resulting solution is concentrated in a vacuum evaporator (under laboratory conditions) and the content of monosaccharides is determined using paper chromatography. The degree of esterification of pectin was determined by the titrometric method and amounted to 74.5 %. This value was determined by the titrimetric method. This indicates a high degree of pectin esterification. The functional groups of pectin were determined on a Perkel-Elmer IR-Fure spectrometer (Fig. 1).
Results and discussing. Obtaining food pectin from food industry waste is cheaper and remains an urgent problem in the food industry. One of the sources of pectin is Heliantus annuus.L family Asteraceae (corn cobs) after extraction of seeds in the oil and fat industry [1].
At present, a corn cobs, after the removal of seeds, is at best used as animal feed, therefore, it is not subjected to industrial processing due to the absence of the latter.
The quality of the pectin depends on the production method. However, under certain conditions of mineral acid hydrolysis-extraction in combination with alcohol precipitation, pectin can be obtained from corn cobs [2].
We have studied the carbohydrate composition of the corn varieties “Salyut” (“Fireworks”) cultivated on the territory of the Gallaaral district of the Jizzakh region, in the Republican scientific research agricultural center [3].
For this, we have developed a method for obtaining pectin using a culture of the fungus Trichoderma harzinium. Its essence lies in the fact that before extraction, the starting material is treated with a culture of the fungus, which, while developing, produces mainly cellulolytic enzymes. So 1 kg of sterilized corn materials was treated with this mushroom culture at a temperature of 36 o C for 10 days. Then, the released pectin substances were extracted with water in a ratio of 1:5 at room temperature for 2 h. The resulting extract was filtered, centrifuged at 5000 rpm for 10 min, and precipitated with 96 % ethyl alcohol at a ratio of 1: 2 for 1 h and clarified in a refrigerator for 3 h at + 40C. The precipitated pectin precipitate was separated, washed 2 times with 80 % alcohol and once with 96 % ethyl alcohol. The precipitate was dried in a thermostat with a temperature of 80–90 o C to a residual moisture content of 15–16 %. The output of pectin was 4 % of the original weight of dry cobs [4].
The resulting pectin is an amorphous substance, light yellow in color, well soluble in water. The purity is high, for example a test for the presence of starch was negative. When mixing 1 % KI and I with a pectin solution, the color of the solution does not change. The relative viscosity of a 1 % solution of pectin was
To determine the monosaccharide composition, samples of pectin and water-soluble polysaccharides undergo complete acid hydrolysis. To 100 g of pectin powder was added 5 ml of 2N sulfuric acid (H 2 SO 4 ) and hydrolyzed for 24 hours. The hydrolyzate was neutralized with barium carbonate salt (BaCO 3 ) and treated with KU-2 + cation. The carbonate ions formed during the treatment with barium carbonate are also neutralized. The resulting solution was evaporated in a vacuum evaporator and the monosaccharide composition was determined using paper chromatography, the results are shown in Table 1.
Table 1
Carbohydrate composition of pectin in corn cobs
|
№ |
Pectin source |
Carbohydrate |
Yield in relation to dry matter, % |
Gal UA |
Gal |
Glс |
Ara |
Xyl |
Rha |
Man |
|
1 |
Corn cobs |
PS * |
4,0 |
+ |
+ |
Trace |
+ |
Trace |
+ |
- |
PS — pectin substances, GalUA — galuronic acid; Gal — galactose; Glс — glucose; Ara — arbinose; Xyl — xylose; Rha — rhamnose; Man- mannose.
To determine free carboxyl groups and the degree of esterification, a titrimetric method was used. The results are shown in Table 2.
Table 2
Physicochemical characteristics of pectin substances from the corn cob
|
Raw materials |
1 % aqueous solution of pectin |
Titrometric data, % |
|||||
|
[
|
|
рН |
К с |
К е |
К о |
|
|
|
PS |
+190 0 |
1,02 |
4,5 |
1,26 |
3,68 |
4,92 |
74,52 |
*
[
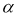
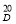
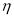
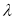
The degree of esterification of pectin substances has been determined. The tabular data obtained by the titrimetric method show that the pectin is highly esterified.
Analyzes of the research results showed that the obtained pectin substance differs from the previous ones in chemical composition, molecular weight, viscosity, specific rotation of light and other constants.
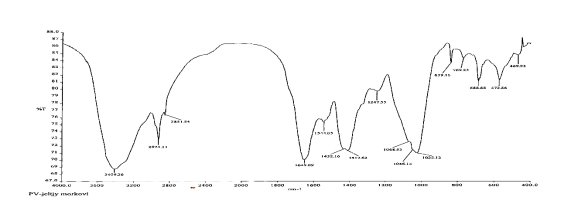
The functional groups of pectin substances were determined using a Perkel-Elmer IR Fourier spectrometer [6].
The IR spectra of pectin substances are characterized by a wide band in the region of 3429 cm -1 , which corresponds to the frequencies of stretching vibrations of hydroxyl OH -1 and CH groups. Absorbances in the region of 2939 cm -1 are characteristic of stretching vibrations of hydroxyls involved in hydrogen bonds. The presence of a carboxyl group, which can be esterified with a methyl or acetyl group or substituted with sodium, gives a number of absorption bands characteristic of pectin substances.
The absorption band at 1740, 1614, 1423 cm -1 represents the stretching vibrations of the carbonyl (C = O) in the COOCH 3 and COOH groups, which indicates the presence of free and esterified carboxyl groups.
The absorption band at 1373 cm -1 clearly shows the presence of methoxyl groups. In this case, we observe the correspondence between the results of titrometric analysis and IR spectra. This result also includes the moment that in the IR spectrum there is an absorption band at 1148 cm -1 , which indicates the presence of an esterified group — methyl.
The absorption band at 1097, 1074, 1048, 1020 cm -1 characterizes the presence of a pyronose cycle (C — O, C — C, C — OH, CH 2 , C — O — C).
The band of emission 816, 892, 917 cm
-1
— triplets of pyronose cycles, indicating the presence of 1
The absorption band at 760 cm
-1
shows the possible presence of
Thus, the results of IR spectroscopy show that this pectin is an esterified polysaccharide.
Conclusion. Due to the high degree of esterification of pectin obtained from corn cobs, its gelling properties are high when used in the confectionery industry, which indicates that high technological efficiency can be obtained in the production of confectionery products with low pectin costs.
References:
- Khalikova D.Kh., Mukhiddinov Z. K., Avloev Kh.Kh., Gorshkova R. M., Khalikova S. Influence of acidity on sunflowers protopektin hydrolysis and microelement composition of its products. //5 th International Symposium on the chemistry of Natural Compounds, May 20–23, 2003, Tashkent, Uzbekistan, P. 247.
- М. П. Филипов. Инфракрасные спектры пектиновых веществ, Штинца, Кишинев. 1978.-С.14.
- Baker A. Food Technology. Chicago № 11 1994 y.
- Walter R.H et al.The Chemistry and Technology of Pektin //Academic Press Inc.Harcourt Brake Jovanovich Publishers.1991 y
- Shin K.S, Kiyohara H, Matsumoto T, Yamanda H.Pectins and Pectinases Elsevier.Amsterdam 1996 y.







