Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) a nitramine explosive, which has contaminated various military sites during its use, storage and manufacturing worldwide. As RDX is a recalcitrant, less soluble and toxic to human beings and other organisms, it is essential to remove the contaminated sites. To date, treatment RDX contaminated water has been tested using a variety of methods such as hydrolysis, electrochemical, microorganism, chemical advanced oxidation processes (CAOPs) or electro-chemical advanced oxidation processes (ECAOPs) do not use non-photo radiation (non-photo-AOPs) [1,2]. However, there are especially limitations on compounds as RDX when degradation of RDX use AOPs showed the speed and efficiency of decomposition of RDX. To date, there has been a lack of publicity regarding the RDX degradation characteristics in water by CAOPs using UV. According to the published researchs, there are little attention about the comparison and evaluation of the conversion efficiency, ECAOPs under conditions of UV radiation.
The purpose of this research is to present the results of the evaluation of removal efficiency of the RDX degradation by ECAOPs based on hydroxyl radical ( OH). The electrified oxidation systems (under the condition of using the non-diaphragm electrolytic cell, with the symbol EO) were investigated as RDX/ EO, RDX/EO- H 2 O 2 ; RDX/ EO-UV and RDX / EO-UV- H 2 O 2 .
Materials and methods
Materials
Crystalline RDX (99 %) were of analytical grade. All solvents (acetonitrile, ethanol, methanol and hexane) used in experiments were of HPLC grade. Hydrogen peroxide (30 %) and all other reagents were of analytical grade. All chemicals were purchased from Merk.
Instruments
RDX were identified and quantified by an HPLC (HP Agilent 1100 series, diode array detector, USA), using a Hypersil C18 column (200 mm × 4 mm). The pH of the solution was adjusted by adding aqueous H 2 SO 4 or NaOH to the desired value, determined by a pH meter (OAKLON, 510 series, USA).
Setup experiments
A lab-scale electrocoagulation reactor constituted a 1 L glass cell that was 10 mm (width) ×12 mm (length) × 15 mm (height) and was set up with two parallel monopolar electrodes with a 2-cm interelectrode distance. The anode and cathode of the electrocoagulation reactor were made of Ti plates with dimensions of 2×7×17 mm. 2 g/L NaCl was used as electrolyte concentration in pH = 6.
Analytical methods
The analytical procedure was described elsewhere [6]. Briefly, RDX was identified and quantified by an HPLC (HP Agilent 1100 series, diode array detector, USA), using a Hypersil C18 column (200 mm 4 mm). The mobile phase consisted of 67 % acetonitrile and 33 % water (v/v) at a flow rate of 0.6 mL/min, with a pressure of 280 bar. The analytical signal was measured at the wavelength of 227 nm. HPLC peaks of RDX, was observed at retention times (t R ) 5.11 min.
The conversion (η, %) of RDX in electrooxidation systems is calculated by the formula:
η = {(C (0)RDX — C (t)RDX )/C (0)RDX }x100 (1) Where is the conversion of RDX (%),C (0)RDX : concentration of RDX at time t= 0; C (t)RDX : concentration of RDX at time t.
Results and discussion
Figure 1 shows the results of the variation in RDX decomposition efficiency over time in the electrochemical oxidation systems: ‘RDX/EO, RDX/EO-H 2 O 2 , RDX/EO-UV, RDX/EO-UV-H 2 O 2 .
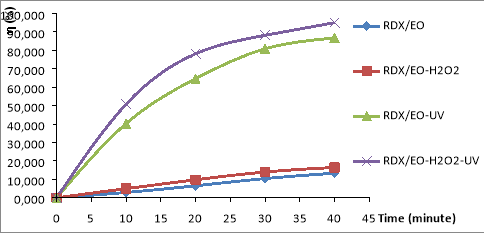
Fig. 1. Removal RDX in the different ECAOPs systems: RDX/EO, RDX/EO-H 2 O 2 , RDX/EO-UV, RDX/EO-UV-H 2 O 2 .(C RDX = 19.22 mg/L, C HMX = 5.8 mg/L, C H2O2 = 14.5 mM, I = 875 Lux, pH = 6)
The results shown in Fig. 1, it is found that the conversion efficiency of RDX and HMX are very low in the RDX/EO and RDX/EO-H 2 O 2 systems. The conversion rate of RDX only increased significantly in RDX/EO-UV and RDX/EO-UV-H 2 O 2 . However, RDX of metabolism is the rule of increasing sequence:
RDX/EO< RDX/EO-H 2 O 2 < RDX/EO-UV< RDX/EO-UV-H 2 O 2
The causes of the above phenomena can be explained as follows: The organic decomposition reaction (signed RHs) can occur in two mechanisms in the electrochemical systems.
- The RHs are directly oxidized by hydroxyl radicals which are formed and adsorbed on the anode surface by oxidation of water on the anode ( OH ad);
- The RHs are indirectly oxidized by OH radicals which are formed in the solution by a result of the reaction between the compounds produced during electrochemical processes.
The direct oxidation is usually carried out in the anode compartment of the diaphragm electrolyser (this oxidation anode is denoted as AEO (RHs/AEO) to distinguish it from the indirect oxidation system carried out in the electrolytic jar without diaphragm EO (RHs/EO). In the RHs/EO and the RHs/EO system, the anodes are usually insoluble as graphite, graphite, platinum, lead, manganese dioxide, RuO 2 , TiO 2 , IrO 2 .... The oxidation of water on the anode in the RHs/AEO system occurs according to equation (3. 4):
H 2 O OH ad + H + + e (1)
The part of the OH ad formed in this reaction will be directly involved in the oxidation of organic pollutants RHs. The rest will react on the anode to form O 2 (3.5):
2OH O 2 + 2H + 2e (2)
Unlike oxidation anode process in the RHS/AEO system, the indirect electrochemical oxidation of RHs in the RHs/EO system is usually carried out in non-diaphragm by OH. The OH are formed in solution by the reaction between the compounds generated during electrochemical process. In this process, the oxidation of water produces the molecular oxygen O 2 at anodic surface (3.6):
2H 2 O O 2 + 4H + + 4e- (3)
This molecular oxygen that is reduced at the catot to form H 2 O 2 (3.7):
O 2 + 2H + 2e H 2 O 2 (4)
Thus, in the indirect electrolytic oxidation systems without H 2 O 2 (eg, RDX/EO systems) or with added H 2 O 2 (eg, RDX/EO-H 2 O 2 systems), there is no involvement of OH in the RDX oxidation. This advanced oxidizing agent is only formed and participates in the oxidative degradation of RDX if the electrolytic solution contains H 2 O 2 , Fe 2+ or UV radiation.
The tests were performed under the condition of using a non-diaphragm electrolytic jar, so it could be considered that the RDX decomposition processes are an indirect electric oxidation processes (EO). Thus, in the RDX/EO system with the absence of UV radiation, RDX are decomposed primarily by conventional oxidation-reduction processes, but absence of OH. In the RDX/EO-H 2 O 2 system, there is also the direct chemical oxidation of RDX by H 2 O 2 . However, H 2 O 2 is a weaker oxidizer than OH, so the addition of H 2 O 2 to the electrolytic solution can not significantly increase the RDX decomposition efficiency. There is significantly increment in RDX with present of OH which was made of the UV-H 2 O 2 effect, for example, RDX/EO-UV or RDX/EO-UV-H 2 O 2 .
In the RDX/EO-UV system, the RDX were decompose by two factors: 1/ The effect of the OH radicals produced by the UV-H 2 O 2 photosynthesic reaction in which H 2 O 2 is product of the oxidation reduction process in solution; 2/ The direct UV radiation effects on the RDX.
The most important of these effects are the effects of OH radicals, so the RDX degradation efficiency of the RDX/EO-UV system is higher than the RDX/EO and RDX/EO-H 2 O 2 system. However, the RDX degradation efficiency of the RDX/EO-UV system is still lower than the RDX /EO-UV-H 2 O 2 system. There is the difference in the initial H 2 O 2 quantity involved in the UV photo — reaction in these two ECAOPs. The H 2 O 2 quantity of the RDX/EO system (without added H 2 O 2 ) is mainly derived from the electrochemical reductive reaction of dissolved oxygen (O 2 ) in solution (reaction 3.4), so the quantity of OH agent formed from the RDX/EO-UV system is significantly lower than the RDX/EO-UV-H 2 O 2 . This is the reason for the lower RDX decomposition efficiency of the RDX/EO-UV system than the RDX /EO-UV-H 2 O 2 system.
However, there is the fact that although the RDX/EO-UV- H 2 O 2 system has the highest activity of the ECAOPs, but the RDX decomposition efficiency of this system is still lower than that of the RDX/UV-Fenton system. The reason of the RDX/UV-Fenton is able to maintain a steady and continuous regeneration of OH that is involved in the oxidation of RDX by the Fenton photo catalysis [16,18,19], while in the RDX/ EO-UV-H 2 O 2 system, the amount of H 2 O 2 is unstable and decreases (resulting in reduced OH amount) during the reaction. The possibility of using the electrochemical photo Fenton effect in this case is not feasible because in these investigations the appropriate electrolytic environment at pH=6 [6], so that when adding Fe 2+ to the solution to occur iron hydroxide precipitate.
Conclusion
The UV radiation and effects such as EO-UV-H 2 O 2 have important influences on the efficiency and rate of decomposition of RDX by electrochemcal oxidation processes. In electrochemcal oxidation processes have been investigated that process with UV-H 2 O 2 effect has the high RDX decomposition rates and the high RDX decomposition efficience. They can treat and clean contaminated water sources, such as RDX compounds.
References:
- Kyung-Duk Zoha, Michael K. Stenstromb Fenton oxidation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) Water Research 36 (2002) 1331–1341
- Heilmann H, Wiesmann U, Stenstrom MK. Kinetics of the alkaline hydrolysis high explosives RDX and HMX in aqueous solution and adsorption to activated carbon. Environ Sci Tech 1996;30(5):1485–92.
- Do Ngoc Khue, Tran Dai Lam, Nguyen Van Cha, Vu Quang Bach, Do Binh Minh, Vu Duc Loi, Nguyen Van Anh. Simultaneous degradation of 2,4,6-trinitrophenyl-N-methylnitramine (Tetryl) and hexahydro-1,3,5-trinitro-1,3,5 triazine (RDX) in polluted wastewater using some advanced oxidation processes. Journal of Industrial and Engineering Chemistry 20 (2014) 1468–1475

