From 30 soil samples taken from many different areas of Da Nang city. We have collected 7 strains of bacteria capable of producing strong antibacterial compounds with E.coli, Salmonella, and B.cereus. In which, V7 strain is the strongest antibiotic production. We studied on physiological and biochemical characteristics of V7 strain and identified strain V7 by sequencing 16S rRNA gene to conclude that this strain belongs to Bacillus velezensis. Optimal conditions for the growth and bioavailability of Bacillus velezensis are with a nitrogen source of yeast and peptone, at temperature 30°C, at pH 7, in time 24 hours.
Key word: antibacterial, Bacillus velezensis, inhibition, E.coli, Salmonella
1. Introduction
Food safety and hygiene are always an concern issue. Currently, dirty food and contaminated food are rampant in the market and threaten consumer health. There are many causes of food poisoning such as microorganisms, raw materials and products containing toxins, the process of processing and preserving food, additives, chemical fertilizers and residues of real protection drugs object...
According to statistics, Vietnam has about 20 to 500 food poisoning cases with 7000–10000 victims and 100–200 deaths each year. The goverment has to spend over 3 billion VND for treatment, testing and investigation to clarify the cause [12].
Therefore, the problem of replacing chemical preservatives with natural products is an effective solution. The study of biological compounds extracted from microorganisms is a relatively feasible approach in the field of preserving agricultural products and in the farming sector. Bacillus is considered a specialized microbiological plant for large-scale production of various types of bioactive molecules [9].
In this study, we conducted the isolation and screening of Bacillus sp. has strong antagonistic activity with pathogenic bacteria, and also finds the optimal environment for them to produce the most active antimicrobial compounds.
2. Materials and Methods
2.1. Isolation of producing bacterial strain
Isolation of bacteria to the bacteriological research method of Nguyen Lan Dung et al [3]. Put 1 gram of soil in a triangle or tube to shake well on the shaker for 30 minutes. It was then serially diluted with concentrations of 10–1 to 10–10 times and each of the dilutions was spread over LB medium at 28°C for 2–5 days, monitor the number of colonies after isolation.
2.2. Physiological and Biochemical characterization of Bacillus sp.
Study on biological characteristics of selected bacterial strains was conducted by the method of Claus and Berkeley (1986) with biochemical tests such as Oxidase, Catalase, Voges Proskauer (VP), spore-forming ability, The Gram stain test, mobility of bacteria [2].
2.3. Selection of strains Bacillus sp. produce antibacterial compounds strongly inhibits with indicator bacteria
From Bacillus strains isolated, Bacillus sp. was increased in LB medium with a shaking rate of 180 revolutions/minute, time of 18 20 hours, determined density 107 CFU/ml. Then divide the suspension into each of the sterile eppendorf tubes of 1.5ml, centrifuge 5000 cycles/min for 30 minutes at 4 ° C to remove cell biomass and collect floating fluid. Collect the supernatant and measure the initial pH, then titrate with 0.5 N NaOH to bring it to pH = 7. This step is to eliminate the effect of acid secreted by bacteria. If alkaline pH, use 0.5 N HCl to titrate to pH = 7.
Test activity by diffusion method on agar plates. Prepare TSA agar plates, sucking 100 µl of indicator bacteria (E.coli, Salmonella sp., Bacillus cereus) diluted in a ratio of 1: 9 with physiological saline, and spread over on the agar surface until the agar surface is dry. Then, punch 2 4 holes, with 5mm diameter on the surface of the agar plate. The perforated holes are about 1 cm away from the edge of petri dish to be able to observe the antibacterial ring later. Using micropipet, suck 150µl of raw antibiotic solution into each agar hole, suck 150µl of sterile distilled water (disinfected at 121°C) into the hole for the control sample. The plates are stored at 2 4°C for 2 hours with the aim of preventing the growth of the indicator strain and enabling antibiotics to penetrate into the agar. Then the plates are incubated at 37oC. After 16 24 hours, read the results.
The antibacterial ability of bacteria is determined by the presence of antibacterial rings around the hole. Diameter of antibacterial area calculated: H = D — d; in which H: diameter of antibacterial area around the hole, D: inhibition area diameter (including hole diameter), d: hole diameter [3].
2.4. Identification of Bacillus sp.
Select the bacteria with the highest antibiotic bioavailability to identify the 16S rRNA sequence.
Culture of microbial biomass was carried out in LB medium on shaking machine for 180 rpm for 16 hours. Total DNA extraction, amplifying the 16S rRNA gene region by PCR with primer pairs:
27F (5’-AGAGTTTGATCCTGGCTCAG-3 ’)
1492R (5’-TACCTTGTTACGACTT-3 ’)
PCR products are purified and sequenced at Phu Sa Biochemical One Member Limited Company (Can Tho). Use BLAST tool on NCBI website to search for similar sequences for results.
2.5. Investigate factors affecting the ability of bacteria to select antibacterial compounds
Experiment 1 Investigate the effects of Nitrogen sources: (NH4)2SO4, NH4NO3, Yeast extracts, pepton at concentration of 0.5 % (w / v) were added to replace nitrogen source in LB. Carrying out Bacillus.sp at 37 ° C for 24 hours. Centrifuge, collect floating fluids and test antibacterial activity by diffusion method on agar plates.
Experiment 2 — Survey the effect of pH:
Raising Bacillus sp. on the optimized medium in experiment 1 and the pH value to 6; 7; 8; 9; 10 at 37 ° C within 24 hours respectively. Centrifuge and collect supernatant and test the activity by diffusion method on agar plate.
Experiment 4 — Investigate the influence of culture time
Raising Bacillus sp. in LB environment was optimal in experiment 1 and optimal pH in experiment 2 and optimum temperature in experiment 3, the time change 12, 24 and 36 hours. Centrifuge, collect supernatant and test the activity by diffusion method on agar plate.
2.6. Statistical analysis
Experimental data are statistically processed by biological statistical method, using data analysis tool of Microsoft excel. Experimental results are indicated by (M ± SE).
3. Results and discussion
3.1. Isolate and preliminarily identify selected strains of bacteria
3.1.1. Isolation of bacteria
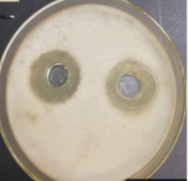
After isolating 30 soil samples taken from many different areas of Danang City on LB environment, 7 strains of bacteria were collected. Based on morphological characteristics when observing colonies, cell shapes, spores under a microscope and based on Bergey's classification key [2]. We identified 7 bacterial strains belonging to the genus Bacillus and temporarily denoted from V1 — V7. Conduct a survey on the ability to produce strong antibacterial compounds with bacteria indicated by diffusion method on agar plates [3]. Results are presented in Table 1.
Table 1
Survey of the ability to produce antibacterial compounds of isolated bacteria strain
|
Symbols of strains |
E.coli |
Salmonella |
B.cereus |
|
V1 |
1.35 ± 0.06 |
1.54 ± 0.0 |
0.9 ± 0.06 |
|
V2 |
1.3 ± 0.14 |
1.1 ± 0.17 |
0.7 ± 0.15 |
|
V3 |
1.62 ± 0.01 |
1.71 ± 0.07 |
1.87 ± 0.03 |
|
V4 |
1.15 ± 0.15 |
1.21 ± 0.17 |
1.31 ± 0.09 |
|
V5 |
0.56 ± 0.03 |
0.67 ± 0.07 |
0.76 ± 0.05 |
|
V6 |
1.35 ± 0.06 |
1.4 ± 0.08 |
1.6 ± 0.06 |
|
V7 |
1.83 ± 0.14 |
1.91 ± 0.17 |
2.06 ± 0.15 |
From the results in Table 1, all strains of bacteria produce antibacterial compounds. These antibacterial compounds can be bacteriocin, hydroxy peroxide, diacetyl [7]. It is these components that create the ability to inhibit the growth of bacteria in the bacteria. Through the experimental results, we found that the strain V7, which is capable of producing high antibacterial compounds, strongly inhibiting the indicator bacteria strains than the other strains. Therefore, we chose strain V7 to perform the next experiments.
3.1.2. Preliminary identification of strain V7 has been selected
Table 2
Biochemical test results of strain V7
|
Bacterial strain |
Size (µm)
|
Shape |
Gram*
|
spore-forming ability |
mobility of bacteria |
Catalase test |
Oxidase test |
VP test |
|
V7 |
0,6 × 1,4 |
The rod cell |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
|
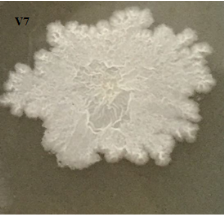
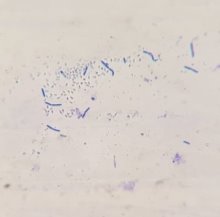
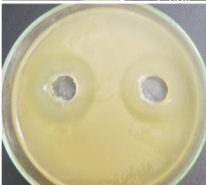
Figure 1: Colony morphology, cell morphology and spore of strain V7 (x100)
Through the survey results in Table 2 and Figure 1, it can be seen that physiological and biochemical properties of strain V7 are suitable for species belonging to genus Bacillus. Every feature is consistent with the reports of Claus and Berkeley [2].
3.2. Identification results by amplifying the 16S rRNA gene region of strain V7
Identify strain V7 by amplifying the 16S rRNA gene region by PCR. PCR products are purified and sequenced at Phu Sa Biochemical Company (Can Tho). The results are shown below.
3.2.1. Amplify the 16S rRNA gene region
Products amplifying the 16S rRNA genome were tested by electrophoresis technique using 0.8 % agarose gel. The results are shown in Figure 2.
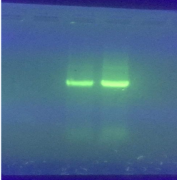
Figure 2. Amplification of the 16S rRNA gene region (Bacillus sp.)
The amplifier product is about 1500bp.
3.2.2. PCR product sequence
Amplified products are read in sequence at Phu Sa Biochemical Company (Can Tho).
Sequence 5'-3 'of 16S rRNA gene (1436bp):
|
GCTATCTGCAGTCGAGCGGACAGATGGGAGCTTGCTCCCTGATGTTAGCGGCGGACGGGTGAGTACACGTGGGTAACCTGCCTGTAAGACTGGGATAACTCCGGGAAACCGGGGCTAATACCGGATGGTTGTCTGAACCGCATGGTTCAGACATAAAAGGTGGCTTCGGCTACCACTTACAGATGGACCCGCGGCGCATTAGCTAGTTGGTGAGGTAACGGCTCACCAAGGCGACGATGCGTAGCCGACCTGAGAGGGTGATCGGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTAGGGAATCTTCCGCAATGGACGAAAGTCTGACGGAGCAACGCCGCGTGAGTGATGAAGGTTTTCGGATCGTAAAGCTCTGTTGTTAGGGAAGAACAAGTGCCGTTCAAATAGGGCGGCACCTTGACGGTACCTAACCAGAAAGCCACGGCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGTGGCAAGCGTTGTCCGGAATTATTGGGCGTAAAGGGCTCGCAGGCGGTTTCTTAAGTCTGATGTGAAAGCCCCCGGCTCAACCGGGGAGGGTCATTGGAAACTGGGGAACTTGAGTGCAGAAGAGGAGAGTGGAATTCCACGTGTAGCGGTGAAATGCGTAGAGATGTGGAGGAACACCAGTGGCGAAGGCGACTCTCTGGTCTGTAACTGACGCTGAGGAGCGAAAGCGTGGGGAGCGAACAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGATGAGTGCTAAGTGTTAGGGGGTTTCCGCCCCTTAGTGCTGCAGCTAACGCATTAAGCACTCCGCCTGGGGAGTACGGTCGCAAGACTGAAACTCAAAGGAATTGACGGGGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGAAGCAACGCGAAGAACCTTACCAGGTCTTGACATCCTCTGACAATCCTAGAGATAGGACGTCCCCTTCGGGGGCAGAGTGACAGGTGGTGCATGGTTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTTGATCTTAGTTGCCAGCATTCAGTTGGGCACTCTAAGGTGACTGCCGGTGACAAACCGGAGGAAGGTGGGGATGACGTCAAATCATCATGCCCCTTATGACCTGGGCTACACACGTGCTACAATGGACAGAACAAGGGCAGCGAAACCGCGAGGTTAAGCCAATCCCACAAATCTGTTCTCAGTTCGGATCGCAGTCTGCAACTCGACTGCGTGAAGCTGGAATCGCTAGTAATCGCGGATCAGCATGCCGCGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCACACCACGAGAGTTGTAACACCCGAAGTCGGTGAGGTAACCTTTATGGAGCCAGCCGCCGAAGGTGGACAATGT |
99 % homology sequence with Bacillus velezensis, allowing to conclude that V7 is Bacillus velezensis.
3.3. The effect of nitrogen source
In the bacteriocin molecule, there is always active protein, bacteriocin biosynthesis always requires organic nitrogen or inorganic nitrogen [5]. In this experiment, Bacillus velezensis, cultured under conditions: 10g / L NaCl, at 37°C for 24 hours, with nitrogen sources: (NH4) 2SO4, NH4NO3, high yeast, strong peptons 0.5 % (w / v) is added to replace nitrogen source in LB. The results are shown in Table 3.
Table 3
Effect of nitrogen source on antibacterial ability of Bacillus velezensis
|
Nitrogen sources |
Antibacterial ring diameter(cm) | ||
|
E.coli |
Salmonella |
B.cereus | |
|
(NH4)2SO4 |
0.7 ± 0.333 |
1.27 ± 0.14 |
0.86 ± 0.14 |
|
NH4NO3 |
0.77 ± 0.167 |
1.49 ± 0.18 |
1.58 ± 0.16 |
|
Yeast extracts |
0.82 ± 0.167 |
1.71 ± 0.21 |
1.0± 0.25 |
|
10g pepton and 5g yeast extracts (LB culture) |
0.95 ± 0.09 |
2.31 ± 0.02 |
2.36 ± 0.04 |
|
Pepton |
0.81 ± 0.01 |
1.21 ± 0.1 |
2.08 ± 0.07 |
The results of Table 3 show that the environment has different sources of nitrogen, the diameter of the ring resolution is different. If using (NH4)2SO4, the diameter of the resolution ring is the smallest (from 0.7 to 1.27cm) because the amount of antibiotic is not much, the antibacterial capacity is not high. This result coincided with the study of Abdel-Mawgoud AM [1] and Makkar RS [8]. In the studies of the two authors, it was found that some strains of B. subtilis could not use (NH4)2SO4 to grow and develop or produce surfactants; however, they can use NaNO3, NH4NO3 or KNO3 [1] [8].
When the nitrogen source is organic, the antibacterial ring increases. Highest value when using 10g high yeast and 5g pepton (LB environment), diameter of 0.95–2.36 cm. This is completely consistent with the growth characteristics of Bacillus velezensis.
According to Phuong Thi Huong's study [5], peptone is the most suitable source of nitrogen for the growth of B. subtilis BSVN15 strain in the nitrogen sources used for investigation with a cell density of 3.55x10 11 CFU / mL. then yeast extracts was 2.56 x10 11 CFU / mL, 2.1 and 1.5 times higher than the control. Inorganic nitrogen sources NH4+, NO3- or urea can be used to supplement the culture medium but cannot completely replace the organic nitrogen source. The amount of peptone suitable for BSVN15 growth is 1–2 % with a density of 4.99x10 11–4.52x10 11 CFU/mL. Nitrogen sources serve as a substrate for bacteria to synthesize the nitrogen-containing compounds necessary for their growth and development.
This result is also consistent with the study of Khuat Huu Thanh (2010) when culturing in a nutrient-assuring environment, strains of Bacillus strains capable of antagonizing pathogenic and harmful microorganisms in shrimp Black tiger [10]. The results of Khem Raj Meena et al 2018 also show that beef-extract is the best source of nitrogen for optimal lipopeptide yield (1852 mg / L) of B.velezensis KLP2016 [6]. Thus, the most suitable source of nitrogen is 5 g of yeast extracts combined with 10 g of pepton (LB medium).
3.4. Survey results of temperature effects of Bacillus velezensis.
Temperature is one of the factors affecting the growth and biosynthesis of antibiotics. The results of survey of the effect of temperature on antibiotic bioavailability of Bacillus velezensis are shown in Table 4.
Table 4
Effect of temperature on antibacterial ability of Bacillus velezensis
|
Temperature (°C) |
Antibacterial ring diameter(cm) | ||
|
E.coli |
Salmonella |
B.cereus | |
|
25 |
1.35 ± 0.06 |
1.54 ± 0.08 |
1.67 ± 0.06 |
|
30 |
1.83 ± 0.14 |
1.91 ± 0.17 |
2.06 ± 0.15 |
|
35 |
1.62 ± 0.01 |
1.71 ± 0.07 |
1.87 ± 0.1 |
|
40 |
1.15 ± 0.15 |
1.21 ± 0.17 |
1.31 ± 0.09 |
|
45 |
0.56 ± 0.03 |
0.67 ± 0.07 |
0.76 ± 0.05 |
From the results Table 4 shows that Bacillus velezensis has antibiotic bioavailability at all five survey temperature levels. In particular, when cultured at 30 °C, the ability to produce antibiotics is highest with an antibacterial diameter of 1.83–2.06 cm. When cultivating Bacillus velezensis at 45 °C, the ability to produce antibiotics is very low, giving antibacterial ring diameter of only 0.56–0.76 cm. According to Khem Raj Meena et al 2018, when analyzing lipopeptide fertility at different growth levels, the maximum lipopeptide yield (2135 mg / L) was observed at 30 °C [6].
3.5. Survey results of pH influence
To investigate the effect of pH on antibiotic bioavailability of Bacillus velezensis strain, is shown in Table 5.
Table 5
Effect of pH on the antibacterial ability of Bacillus velezensis
|
pH |
Antibacterial ring diameter(cm) | ||
|
E.coli |
Salmonella |
B.cereus | |
|
6 |
1.12 ± 0.08 |
1.05 ± 0.03 |
1.17 ± 0.21 |
|
7 |
1.83 ± 0.18 |
1.92 ± 0.04 |
2.06 ± 0.18 |
|
8 |
1.63 ±0.14 |
1.78 ± 0.03 |
1.81 ± 0.18 |
|
9 |
1.07 ± 0.08 |
1.40 ± 0.03 |
1.51 ± 0.21 |
|
10 |
0.5 ± 0.14 |
0.3 ± 0.03 |
0.0 |
Table 5 shows that Bacillus velezensis has the ability to synthesize antibiotics in a fairly wide pH spectrum (from 6–10), the optimal pH for the biosynthesis of antibiotics of this strain is pH 7, diameter antibacterial ring from 1.83–2.06cm. This is consistent with the results of the study of lipopeptide production of B. velezensis KLP2016 showing that pH 7 of the medium supports maximum lipopeptide production (2506mg / L) [6]. When the pH from 10, the ability to produce antibiotics is very low or does not produce. Because, when the pH rises, it will break down the plasma membrane, damage the microbial cells, and also change the electrolyte state of the nutrient molecules, lowering their ability to use them. Microbiology [3]. Therefore, the amount of antibiotic borne is less, leading to a decrease in antibacterial ring diameter.
3.6. Survey results affect culture time
Culture of Bacillus.sp bacteria in LB environment supplemented with high yeast and pepton and pH7, changing time is 12, 24, 36 and 48 hours. Centrifugal, collected supernatant and tested activity by diffusion method on agar well. The results are shown in the following tables and figures.
Table 6
Effect of time on antibacterial ability of Bacillus velezensis
|
Time (hour) |
Antibacterial ring diameter(cm) | ||
|
E.coli |
Salmonella |
B.cereus | |
|
12 |
1.16 ± 0.05 |
1.26 ± 0.14 |
1.41 ± 0.13 |
|
24 |
1.83 ± 0.07 |
1.91 ± 0.11 |
2.06 ± 0.17 |
|
36 |
0.8± 0.05 |
1.0 ± 0.15 |
0.67 ± 0.18 |
|
48 |
0.6± 0.08 |
0.72 ± 0.01 |
0.3 ± 0.21 |
|
|
|
|
|
E.coli |
Salmonella |
B.cereus |
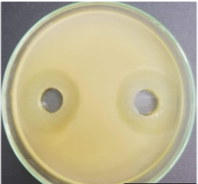
Figure 3. Antibacterial ring diameter of Bacillus velezensis with strains indicated for 24-hour culture
From the results of Table 6 and Figure 3, the culture time significantly affects the antibacterial ability of Bacillus velezensis. After 24 hours of culture, the bacteria thrive, the amount of antibiotics generated is high and the antibacterial ring diameter with the indicator is the largest of 1.83–2.06 cm, three times higher than the time of 10 hours. However, up to 36 hours, the antibacterial ring diameter decreased sharply by 0.67–1.1cm. The decrease in antibiotic activity in culture media can be explained by various reasons. When prolonging the culture period, the environmental composition changes due to the formation of new compounds in the metabolism of bacteria, the content of nutrients decreases... causing bacterial cells to degrade, antibiotics are inactivated [4].
When compared to Todorova (2010), the results are similar. According to Todorova's study, the Bacilus subtilis strain grows at maximum after 20 hours of culture. Antimicrobial activity also increased with bacterial growth and peaked at 24 hours of culture, gradually reduced when cultured at 36 hours [11].
4. Conclusion
Through the above research results, I have drawn some following conclusions:
− From 30 soil samples taken from many different areas of Da Nang city, 7 strains of bacteria have been able to produce strong antibacterial compounds inhibiting bacteria. In which, V7 has the ability to produce the strongest antibiotic. Research on physiological and biochemical characteristics of strain V7.
− Results of identification of strain V7 by sequencing 16S rRNA gene showed that this strain belongs to Bacillus velezensis.
− Optimal conditions for the strongest growth and bioavailability of Bacillus velezensis are in LB medium with 10 g of pepton and 5 g of high yeast, temperature of 30 ° C and pH 7 in 24 hours.
References:
- Abdel-Mawgoud AM, Aboulwafa MM, Hassouna NAH. Optimized production of Surfactin by Bacillus subtilis isolated BS5. Biotechnology Appl. 2008; 150: 305
- Claus D. and Berkeley CW. The Genus Bacillus. In: Sneath PHA ed. Bergey’sManual of Systematic Bacteriology. Williams and Wilkins,. Baltimore; 1986:
- Nguyen Lan Dung, Doan Xuan Muy, Nguyen Phung Tien, Dang Duc Trach, Pham Van Ty (1972), Some methods of microbiological research, Hanoi Science and Technology Publishing House.
- Do Thi Hien, Investigation of the factors affecting the ability of Bacilus subtilis to synthesize Bacteriocin and test antagonistic ability on vibro spp. Thu Dau Mot University Journal, S. 37 (2018): pp 14–24
- Phuong Thi Huong, Vu Van Hanh, Selection of fermentation conditions for the growth of Bacillus subtilis BSVN15 in production of probiotic preparations in livestock. Biotechnology Journal, vol. 16, no. 1, 2018, p 167 –172
- Khem Raj Meena, Tanuja Tandon, Abhishek Sharma, Shamsher S. Kanwar *, Lipopeptide antibiotic production by Bacillus velezensis KLP2016, Journal of Applied Pharmaceutical Science Vol. 8 (03), pp. 091–098, March, 2018
- Ma, C. W., Y. S. Cho and K. H. Oh, 2009. Removal of pathogenic bacteria and nitrogens by Lactobacillus spp. JK-8 and JK-11. Aquaculture. 287: 266–270.
- Makkar RS, Cameotra SS. Produces surfactants with a thermophilic Bacillus subtilis strain. Biotechnology J Ind Microbiol. 1997; 18:37
- Roongswang N, Washio K, M. Diversity Morikawa of nonribosomal peptide synthetases involved in biosynthesis of lipopeptide biosurfactants. Int Mol Sci, 2011; 12: 141–172.
- Khuat Huu Thanh (2010). Research and application of biotechnology to complete BIO-TS3 preparations can increase the resistance of shrimp in intensive tiger shrimp farming. Hanoi University of Technology Journal
- Todorova, S. (2010). Specifies and test the bacteria of Bacillus subtilis of the gradients from country. World J Microbiol Biotechnol. Vol 26 (7): pp.1207–1216
- http://www.vfa.gov.vn/