The present study relates to methods for processing copper slags and can be used in copper metallurgy in order to reduce metal losses from slags [1].
The aim of the study is to increase the degree of copper extraction in the application of low-waste technology, reducing overall costs in the production of copper. Along with this requires the extraction of metal cord from waste tires [2].
This goal is achieved by the fact that instead of clinker, which acts as a reducing agent and instead of pyrite-containing tailings from Lead-Enrichment Factory, which play the role of sulfidization, waste tires are used, containing a large amount of hydrocarbon and sulfur, which play the role of reducing agent and sulfidization [3].
The composition of the materials of the proposed study is illustrated in tables, where table 1 presents the chemical composition of the converter slag and table 2 presents the chemical composition of the waste tires.
Table 1
The chemical composition of the converter slag of AMMC (Almalyk Mine-Metallurgical Combine)
|
Chemical composition, % | ||||||||
|
№ |
Сu |
Fe (total) |
SiO2 |
Al2О3 |
CdO |
Fe3О4 |
S |
MgО |
|
1 |
2,4 |
45,3 |
20,2 |
1,6 |
1,7 |
27,3 |
1,3 |
0,4 |
|
2 |
2,51 |
48,8 |
22,4 |
1,8 |
1,44 |
19,1 |
- |
- |
|
3 |
3,05 |
48,7 |
21,2 |
3,1 |
0,56 |
22,02 |
- |
0,56 |
Table 2
The chemical composition of waste tires,%
|
№ |
Chemical composition | ||||
|
С |
H |
S |
O |
Others | |
|
1 |
83,75 |
7,58 |
4,62 |
2,31 |
1,74 |
|
2 |
78,61 |
7,11 |
8,35 |
1,57 |
4,35 |
|
3 |
74,33 |
6,72 |
12,43 |
2,82 |
3,7 |
The main criteria for minimizing the loss of copper with slags is that the reducing agent and the sulfidizing agent are located at the same source and reducing the mass of the slag formed. For the smallest possible slag yield, you need to add the optimal amount of tire waste.
The processing of copper slags using waste tires is carried out as follows: the process consists of two stages. At the first stage, the preliminary slag depletion is performed. Before it is drained from the converter into the bucket, waste tires must be loaded into the bucket.
Crushed tires, pre-loaded into the slag ladle in the amount of 3 to 5 % by weight of slag (Fig. 1). When slag is drained, the car tires heat and decompose, which is accompanied by the formation of a reducing agent — solid carbon and a gaseous reducing agent — hydrogen. In addition, sulfur in the composition of the tire contributes to the sulfidation of oxidized copper compounds and their transition to the bottom phase. The gases released during these reactions contribute to the coalescence of the fine drops of matte.
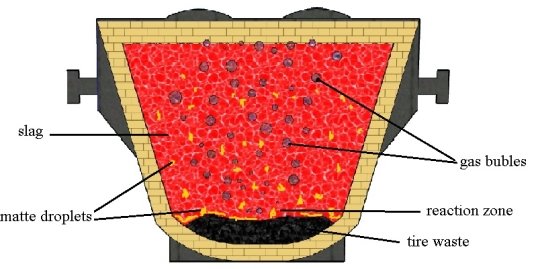
Fig. 1. Scheme of pyrometallurgical processing of copper slag into the ladle
After the slag is poured from the converter into the ladle, the following chemical reactions take place (at 1100–1200 oC):
(C4H6S0,25)n → 4nC + 6nH2 + 0,25nS
C + 2S → CS2
S + H2 → H2S
Cu2Oslag + C(H2) → 2Cumetallic + CO(H2O)gas ↑
Fe3O4 + C(H2) →3FeO + CO(H2O)gas ↑
FeO + C(H2) → Femetallic + CO(H2O)gas ↑
Fe3O4 + Femetallic → 4FeO
3FeO + 4C → Fe3C + 3COgas ↑
Fe3O4 + CО → 3FeO + CO2 gas ↑
С + СO2 → 2COgas ↑
С + Н2О → СОgas↑ + Н2 gas↑
The chemical reactions occurring are exothermic, therefore, when performing work, the temperature of the slag does not decrease (1100–1200 °C). At 1000–1100 °С, hydrocarbons, which are present in the composition of car tire waste, decompose to form carbon and hydrogen. Both reagents reduce copper and iron from their oxides. Sulfur present in the composition of automobile tire waste sulphide copper and iron oxides:
2Cu2Oslag + 3S → 2Cu2Smatte + SO2 (gas) ↑
2Fe3O4 + S → 6FeO + SO2 (gas) ↑
2FeO + 3S → 2FeS + SO2 (gas) ↑
Cu2Oslag + FeS → Cu2Smatte + FeO
2Cu2Oslag + CS2 → 2Cu2Smatte + CO2 (gas)↑
Сu2Oslag + H2S → Cu2Smatte + H2Ovapor↑
After the reduction of magnetite, the density and viscosity of the slag is decreased and this allows the formation of a slag-matte phase in the system.
Comparative experiments on settling the slag in the ladle after releasing the Reverberatory furnace in the presence of matte without mixing showed that in 30 minutes the copper content in the slag decreased by 7–15 %. The copper content in the sulfide suspension at the sludge almost did not change and significantly exceeded the copper content in the bottom matte.
The results of slag purging Convertor confirmed the results obtained in the laboratory and showed the fundamental possibility of the depletion of slags from the converting by the proposed method.
Stirring the smelting products inside a melting unit is a technique known in metallurgy. Nitrogen, natural gas, or an air-oxygen mixture are used as a mixing reagent. The amount of gaseous reagent required for mixing the smelting products is determined by the required mixing intensity. In the industrial implementation of the process, the amount of gaseous reactant will also be determined by the degree of dilution of exhaust gases by combustion products or neutral gases, and in the case of mixing with oxidizing gases, by the degree of additional enrichment of the matte obtained.
The study can be useful when developing methods for the depletion of slags from various autogenous processes. The results of laboratory analysis of the chemical composition of matte and slag are shown in Tables 3 and 4.
Table 3
The chemical composition of the resulting copper matte into the ladle
|
№ |
Time of mixing, min |
Matte forming, % |
Content of copper matte, % |
Extraction of copper into the matte, % |
Slag forming, % | ||
|
Сu |
Fe |
S | |||||
|
1 |
10 |
10,31 |
14,9 |
70,6 |
6,07 |
69,8 |
88,2 |
|
2 |
20 |
10,9 |
12,7 |
44,8 |
5,6 |
60,2 |
87,7 |
|
3 |
20 |
10,4 |
15,3 |
64,3 |
5,1 |
69,1 |
87,9 |
Table 4
The chemical composition of the slag after pyrometallurgical processing into the ladle
|
№ |
Time of mixing, min |
Chemical composition of slag, % | |||||
|
Cu |
Fe |
SiO2 |
CаО |
S |
Zn | ||
|
1 |
10 |
0,89 |
43,7 |
22,0 |
1,48 |
2,56 |
0,37 |
|
2 |
20 |
1,02 |
39,4 |
24,7 |
1,29 |
2,7 |
0,09 |
|
3 |
20 |
0,79 |
42,6 |
23,8 |
1,3 |
2,1 |
0,10 |
At the second stage, products obtained in the optimal amount are processed in a Reverberatory furnace or in a Vanyukov furnace.
References:
- A. A. Yusupkhodjayev, Sh.T. Khojiyev. Methods of decreasing of Copper loss with Slag in Smelting Processes// International Academy Journal Web of Scholar. Kiev, March 2017, № 2(11), Vol. 1, PP. 5–8.
- Khojiev Sh.T. Pyrometallurgical Processing of Copper Slags into the Metallurgical Ladle // IJARSET. Vol. 6, Issue 2, February 2019. pp. 8094–8099.
- Yusupkhodjaev A. A., Khojiev Sh.T., Valiev X. R., Saidova M. S., Omonkhonov O. X. Application of Physical and Chemical Methods for Processing Slags of Copper Production // IJARSET. Vol. 6, Issue 1, January 2019. pp. 7957–7963.

