The article examines breakthrough technologies: SMA PROGRESS/Central Research Institute “WAVE” crypto labels label system and blockchain as a service (BaaS) for pharma industry.
Keywords: blockchain, counterfeit, cryptographic, labels, reader, technology, pharmaceutical industry, ERP-system, mobile crypto terminal.
The QuintilesIMS Institute predicts that the pharmaceutical market will reach nearly USD $1.5 trillion by 2021. U.S. market growth will slow by half in 2016 to 6–7 % from 12 % in 2015, and is forecast to average 6–9 % through 2021. A worldwide increase of counterfeit medicine sales by 90 % over five years was estimated by The World Health Organisation (WHO), with a worldwide sale estimate of $75 Billion in 2010. Counterfeit medicines pose a significant danger to public health in developing as well as developed countries. Counterfeit medicines may be distributed through different channels such as government and private hospitals, pharmacies or other legitimate or illegitimate distributors. Licensed distributors, pharmacists, health care providers or patients may be unable to detect or differentiate between counterfeit and genuine medicines. It has been difficult to assess the extent of the problem of counterfeit medicines in many settings because of the lack of resources/skills to detect counterfeit medicines, the absence or weak medicines regulatory systems, the different definitions of counterfeit medicines in different countries worldwide, as well as the variations in the distribution systems [8].
Main part
Europe and the United States are considered the world leaders in the safest pharmaceutical markets for patients. New sales channels such as the internet as well as global and more complex manufacturing and distribution channels make it increasingly easier for pharmaceutical counterfeiters to market counterfeits directly or to infiltrate the supply chain. With the EU's counterfeit protection directive 2011/62/ EU Falsified Medicine Directive, (FMD) and the Drug Supply Chain Security Act (DSCSA), the European Union and the United States have adopted a comprehensive legal framework for greater patient protection and set the stage for even greater pharmaceutical security. On 27 November 2013 the Drug Quality and Security Act (DQSA) came into force (H.R. 3204). The Drug Supply Chain Security Act (DSCSA) which is Title II of the DQSA, names the compulsory steps on the way to the implementation of an electronic, interoperable system that allows the identification and tracking of marketed prescription drugs in the US. By 2023, the DSCSA will be well on the way to enabling serialized traceability of individual packages of drugs throughout the supply chain. EU & US-Model verification system see table 1 and fig.1.
Table 1
|
EU-Model: End-to-end verification system by means of a unique identifier plus tamper protection. Mandatory examination of both security features when the drug is dispensed. The aim of the FMD is to ensure the identification and authenticity of a drug by an end-to-end checking system with the use of security features. Pharma manufacturers provide the packaging with the two mandatory security features — a unique identifier and a device for tamper protection — at the beginning of the supply chain. At the time of the drug's being dispensed to the public checking takes place to verify the authenticity and integrity of the security features. The verification of the authenticity of the unique identifier ensures that the drug comes from a legitimate producer and has not yet been placed on the market with this unique identifier. The integrity of the anti-tamper device shows that the packaging has not been opened or changed along the supply chain. The FMD has no documentation requirements for individual transactions along the supply chain [11]. |
US-Model: Product verification plus documentation of buying and selling along the supply chain. No verification of serial numbers when the drug is dispensed to the patient. The aim of the DSCSA is to improve traceability throughout the US supply chain. Potentially dangerous products should be more easily recognized and identified and recalls in the case of defective products can be performed more efficiently. The DSCSA addresses every step in the supply chain and tracks all stages on the way from the pharmaceutical manufacturer to the pharmacist. Full traceability is provided down to the level of the product's sales packaging for this implementation through to 27 November 2023. For prescription drugs the supply chain extends from the pharmaceutical manufacturer to the doctor only through authorized trading partners who are licensed or registered with a state or federal authority [11] |
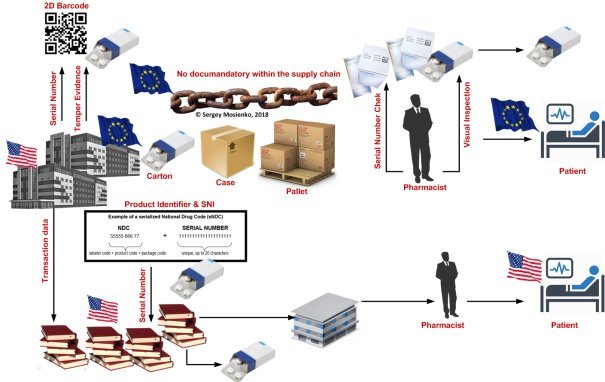
Fig. 1. EU and US Model verification system
EU-Model: End-to-end verification system by means of a unique identifier plus protection. Mandatory examination of both security features when the drug is dispensed. US-Model: Product verification plus documentation of buying and selling along the supply chain. No verification of serial numbers when the drug to the patient.
FMD: Tamper Protection. To comply with the FMD pharmaceuticals manufacturers at the beginning of the supply chain have to ensure a means for protecting the packaging from being tampered with (Tamper Evidence). Its integrity when dispensing the drug documents that the packaging has not been opened along the supply chain. The DSCSA does not provide such a means of tamper protection. DSCSA: Documentation of the Supply Chain. An essential security component of the DSCSA is the precisely logged traceability of the drug across the entire supply chain. Infringements of the documentation requirements and violations of the registration and licensing regulations stipulate firm sanctions by fines, suspensions, license withdrawals and even imprisonment. The FMD in the EU prescribes no documentation by individual locations in the supply chain and focuses on testing for counterfeits when dispensing the drug [11]. FMD: Unique Identifiers (Data Matrix Code). The verification of the authenticity of the unique identifier stored in the Data Matrix code when dispensing ensures that the drug originates from the legal manufacturer or packer and has not been deleted from the central database. The standard check of the Data Matrix code on dispensing is an essential security component of the FMD. The DSCSA provides no comparable procedure when dispensing the drug and focuses on the protection of the supply chain. Tests are carried out on suspected counterfeits.
While in the US serialization should allow all the activities of a preparation over the entire value chain to be reproduced — a check on the authenticity of the product is made only in case of suspicion- Europe takes another route: Here the focus is on the verification of the authenticity of a product prior to its being delivered to the buyer, while the route to the buyer is not fully documented. Both have their advantages and disadvantages. The 2D barcode stipulated by the FMD is used for verification of the unique identifier. In accordance with the FMD manufacturers are also obliged to print the product code and serial number in a human-readable format. The DSCSA defines a so-called Product Identifier which is to be attached by manufacturers of prescription drugs at the latest from 27 November 2017, as well as by repackers onto individual packs and cartons from 27 November 2018. What Information is Provided in the Product Identifier? The Product Identifier includes the National Drug Code (NDC), a serial number, the lot number and expiration date. It includes a machine-readable tag as a 2D Data Matrix code at pack level, and a linear or 2D Data Matrix code at carton level. Several countries are now mandating that companies confirm the authenticity of their product by creating a «pedigree» that vouches for a medication's origin and how it has subsequently been handled. The U. S. FDA (United States Food and Drug Administration) has recommended that pharmaceutical companies start using radio frequency identification technology (RFID) as a means of better tracking drugs. Several pharmaceutical companies are experimenting with RFID and optically variable devices (OVDs) or at least using bar codes or other technologies such as web portals that can help track and authenticate the drugs. Some companies are also testing holograms, QR-code (Quick Response Code), color-shifting inks and watermarks that can help them authenticate the package and actual pills. Others are experimenting with using inks or dyes and some are already using tamper-resistant packaging tape on some of their products [8]. All listed technological solutions (holograms, OVDs, RFID, QR-code) currently being tested are expensive not very effective for protection against counterfeit drugs. A authenticity solution of their drugs based on the holograms, OVDs, bar code, QR-code no future since they do not have the ability to record the “history” or «pedigree» of the passage of drugs from hand to hand. Today people want to know the whole drugs history, from its composition of the treatment that the manufacturer uses, the date of manufacture of the drugs, the time of storage in the warehouses of the distributors, and the time and date of transportation of the drugs by the transporter companies. Today it is not difficult to forge a hologram, OVDs, bar code, QR-code. Нologram’s, OVDs, bar code, QR-code technologies used for labeling drugs can not be connected to new blockchain technology. The chips used for RFID or NFC technology can be cloned without hindrance by hackers. The problem of cloning RFID/NFC chips by hackers has been devoted to a large number of scientific papers. Alternative technologies offer different levels of applications and protection see table № 2.
Table 2
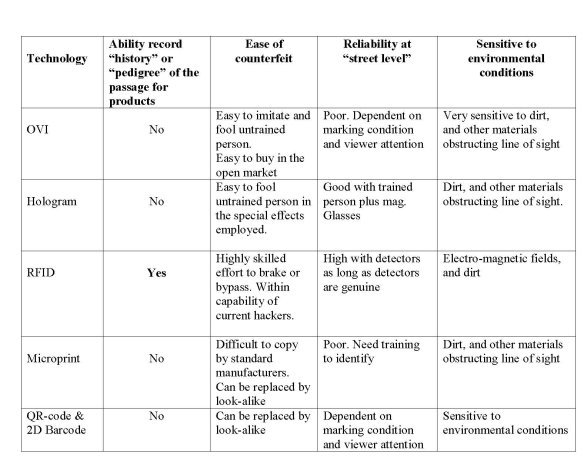
I don’t think you can believe the hologram’s, OVDs, 2D Barcode, QR-code on the drug boxes today. Fraudsters have long mastered the hologram’s, OVDs, 2D Barcode, QR-code technology’s, which is why the make a huge profit from the sale o counterfeit drugs. Representatives of the WHO have long been time to seriously get acquainted with the protection technologies that can be used for medicines. I want to stop the killing of people from counterfeit drugs. Can this be done today? I think that this can be done with the help of innovative SMA PROGRESS/ Central Research Institute “WAVE” Crypto Labels Systems (CLS) and blockchain technology. The CLS and blockchain solution to this issue works together with the Good Distribution Practice (GDP) regulation adherence previously outlined. The blockchain would enable consumers to track the path of their product throughout the entire supply chain and therefore verify its authenticity. This would be made possible by packages being logged every time they change hands. A crypto labels would be scanned which would enter the transaction onto the immutable blockchain. A product could be therefore scanned at any time by a customer or a producer to view the entire distribution history on the blockchain. It is fully transparent and cannot be changed, and all inputs will only be possible by trusted parties. Another benefit of the system is that if there is disruption in a part of the supply chain, the public ledger of the blockchain provides an efficient and reliable means to track where the issue arose and who was in possession of the shipment at the time. The drug supply chain is a very complicated process involving many transactions, and throughout the entire process adhering to strict regulations is of upmost importance. Currently, manufacturers have little transparency of the supply chain process to track authenticity, which is an issue. SMA PROGRESS/Central Research Institute “WAVE” the Crypto Labels System (CLS) includes a the Crypto Labels Reader (CLR) with the Cryptographic Labels (CL), the Mobile Crypto Terminal (MCT) and Amazon Web Services (AWS) Blockchain for Hyperledger Fabric (see Fig. 2). AWS Blockchain Templates provides a fast and easy way to create and deploy secure blockchain networks using the Hyperledger Fabric open source frameworks. The secure blockchain networks can be seamlessly integrated into existing ERP-software systems of pharmaceutical companies.
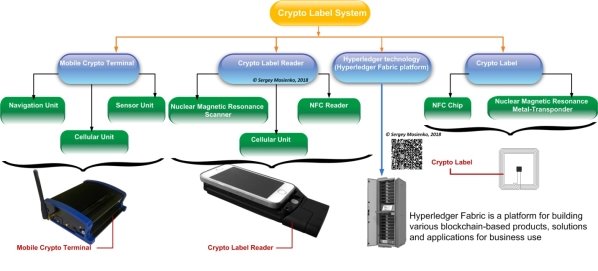
Fig. 2. SMA PROGRESS the crypto label system composition
The crypto labels reader
SMA PROGRESS/Central Research Institute “WAVE” the CLR [1, 6] contains a Nuclear Magnetic Resonance (NMR) scanner and RFID or NFC (Near Field Communication) reader, cell modem and microcontroller (see Fig.3). The NMR scanner at the CLR for authenticating and/or identifying of the CL for drugs comprise a units generating either continuous or pulse, either modulated or non modulated emitted radiation in the radio frequency band, including a generator of continuous or pulse modulated or non modulated radio frequency signal and an emitting probe head or coil, transforming it into electromagnetic radiation, and a system for detection of the re-radiation emitted by the resonant substance in response to the radio frequency radiation, including receiving probe head or coil and detection device with a registration device determining presence of the re-radiation from the resonant substance.
The crypto label
SMA PROGRESS the СL’s [7] (see Fig. 4) consist the RFID/NFC-chip, antenna and insulating material with magnetic resonance metal-transponder (MRMT) on which materials are deposited nuclear magnetic resonance in ferromagnets, or antiferromagnets, or ferrimagnets, or nuclear quadrupole resonance, or very low field electron spin resonance, or said resonance phenomenon is due to electric/magnetic dipole or tunnel transitions between Stark-Zeeman sub levels, or any combinations or aforementioned phenomena.
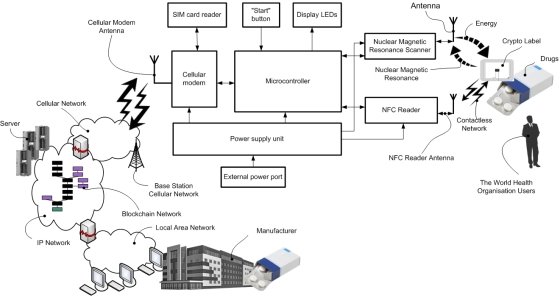
Fig. 3. SMA PROGRESS crypto labels reader block diagram [1]
The СL’s, tamper-proof digital fingerprints, to be embedded into products, or devices of products, and linked to the blockchain. For example MgFe2O4 nanoparticles can be used as the MRMT. Powder with MgFe2O4 nanoparticles can be obtained by qlycine-nitrate combustion in accordance with the metod described in the journal Pharmazine [10]. We have obtained a lot of the MRMT with the required magnetic characteristics having nuclear magnetic resonance properties. To detect nuclear magnetic resonance for each magnetic resonance metal-transponder nanoparticle, we have developed a universal NMR scanner. We deliver to each customer of our the CLS individual the NMR scanner with allow to read only individual the crypto labels containing the magnetic resonance metal-transponder. It insulating be noted that our unique the crypto label contains insulation. The isolation is placed between the chip and the NFC antenna pins, that is, actually be between the chip and the NFC antenna pins. The insulation consists of a double-sided polyethyelene tape, while the upper and lower sides of the polyethyelene tape are coated with a glue containing a the magnetic resonance metal-transponder having the properties of nuclear magnetic resonance. These fingerprints can take many forms such as tiny NFC-chips, but when they are tied to a blockchain, they represent a powerful means of proving a pharmaceutical industry authenticity. These crypto labels pave the way for new solutions that can combat fraud and protect consumers. The cell modem transmits the information data received from the CL’s via the base stations of the cellular network to the distributed database servers blockchains technology. The blockchain gives internet users the ability to create value and authenticates digital information the CL’s. Developing digital identity standards is proving to be a highly complex process.
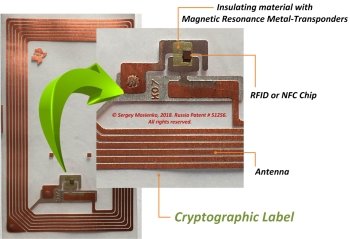
Fig. 4. SMA PROGRESS crypto label [6]
The mobile crypto terminal
SMA PROGRESS/Central Research Institute “WAVE” the mobile crypto terminal (MCT) [2,3,4,5] (see Fig.5) consist the RFID/NFC reader, NFC antenna, GNSS (Global Navigation Satellite Systems) receiver, inertial navigation system (INS), cellular modem, microcontroller, flash memory and security chip. The MCT has a flash memory in which the readings from the GNSS Receiver and connected sensors are recorded. All the request of the users of the blockchain network, the MCT transmit encrypted data the network servers.
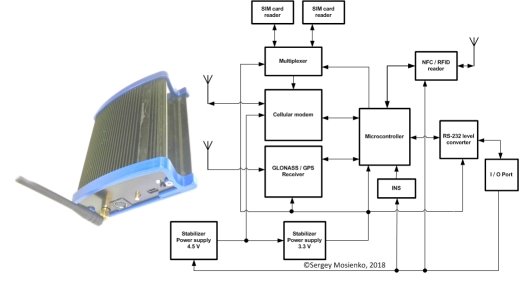
Fig. 5. SMA PROGRESS the mobile crypto terminal [2, 3, 4, 5]
Encrypted data are information from the crypto label, which is read by the NFC Reader, the data location and data from sensors that are connected to the MCT. The MCT extremely necessary for transportation and special control of thermolabile immunobiological drugs. One of the main aims for quality preserving of drugs is providing of appropriate temperature regime during all stages of turnover. According to WHO data about 25 % of vaccines are delivered spoiled because of violation of transportation and/or storage regimes, moreover, they are not fixed by the control equipment. It is obvious, that application of such drugs can cause direct health hazard and even death for patients. Thermal recording devices or the MCT are deprived the abovestated lacks and for today are considered as more effective temperature monitors. The MCT provides accumulating in its own flash memory information about 100 000 or 300 000 events within temperature ranges –40 °C ÷ +85 °С with relative error not more ±1 °С. Obtaining of the results which have been saved up by the MCT, and also task of new values of adjusting parameters for continuation of their work are performed by means of the usual personal computer or servers blockchain networks. Huge advantages of crypto labels system and NFC/RFID technology is possibility of remote monitoring of cargo condition, especially temperature regime of transportation.
How is it working?
Each drugs of interest should be marked by a certain data carrier — crypto labels. The crypto labels contains information on an drugs, its manufacturing features (origin, batch, time stamp) and destination (where the object should be delivered etc.). The marking occurs at manufacturing facilities, in distribution centers etc. The latter facilities form corresponding data base which stores precise conformity of drugs and data attributed to these drug at marking. At inspection points the crypto labels readers read information from the crypto labels and transfer this information to corresponding data bases for checking its validity and providing tracking data. It is very important that information read from the crypto labels will not be distorted/counterfeited over all network transactions. Of course, databases themselves must be highly secured. The blockchain technology provides unique uncompromised abilities to protect secure data transfer and processing. Thus, blockchain perfectly protects this part of the crypto labels system. However, all smart blockchain technologies will be absolutely useless in the case when counterfeiters will compromise/fake the data carrier (crypto labels) itself. Let us suppose a counterfeiter will duplicate the adhesive crypto label attached to an authentic drug, and then attach it to the faked drug. Indeed, both crypto label (data carrier) and data base contain full information on authenticity of that drug. The crypto labels reader in the inspection point will read data form an unknown drug and then secure transfer it to data base using advanced blockchain technology. And inform the inspector that the drugs is authentic. Sounds good, doesn’t it? Let us suppose that next time another inspector will read another drug carrying the same data. And then secure transfer it to the data base. Which response will it get from the data base? Is that drug authentic? Data base will inform on duplicated data (the drug was checked before) and put the authenticity of this drug under question. May we call the second drug not authentic (or having other destination etc.)? Which one of these two (three, four etc.) of these drugs was really authentic? Nobody knows! That is why the blockchain technology only cannot provide real crypto labels system. It is absolutely obvious that the data carrier must be physically protected from duplications/imitations. The crypto labels reader at the inspection point must be confident that the data carrier on an drug is original, i.e. not faked. Thus, the reading device (the CLR) must have some tools for machine recognition of the authenticity of data carrier (the CL's). Nuclear magnetic resonance (NMR) scanner at the crypto labels reader provides perfect tools for such recognition. Indeed, magnetic resonance metal-transponder (MRMT) provides machine readable invisible tag for physical anti-counterfeiting protection crypto labels (crypto chip) of drugs. Each drug (data carrier) is reliably protected from any unauthorized duplication. For working 10 years in anti-counterfeiting and brand protection business, magnetic resonance metal-transponder having been never compromised, i.e. totally faked or somehow imitated. Magnetic resonance metal-transponder for crypto labels (crypto chip) in combination with blockchain perfectly matches all targets of crypto labels system.
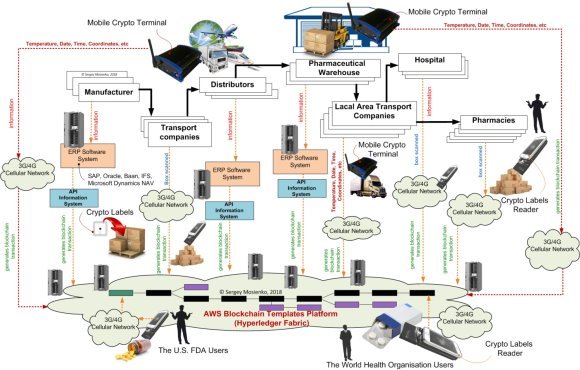
Fig. 6. SMA PROGRESS Pharma Crypto Labels System Architecture
In the proposed version of crypto labels system each data carrier it tagged by magnetic resonance metal-transponder for crypto labels. The crypto labels reader in the inspection point first checks the crypto labels (data carrier) for its authenticity by magnetic resonance metal-transponder for crypto labels. It takes a couple of seconds or even less. In the case the data carrier (crypto labels) is authentic; the crypto labels reader (inspection device) reads data and then securely transfers them (together with the attribute “authentic”) through network to data base. In the case the crypto labels is found to be faked, the crypto labels reader may inform authorities and/or data base on appearance and location of the faked/compromised drug (object). The cell modem transmits the information data received from the CL’s via the base stations of the cellular network to the distributed database servers blockchains technology. The blockchain gives internet users the ability to create value and authenticates digital information crypto labels. Leveraging blockchain is not about replacing well-established forms of supply chain interactions, such as Enterprise Resource Planning (ERP) software systems (see Fig.6), for example SAP, Oracle, Microsoft Dynamics NAV or IFS. Rather, as pharma organizations implement new supply chain technologies, for example Internet of Things (IoT) technologies for improved logistics processes monitoring, blockchain will be used provide a synthesized record of information flows. This level of shared visibility will offer drugs plant an opportunity to optimize multi-party supply chain processes drugs.
Conclusions
Developing digital identity standards for pharma is proving to be a highly complex process. Technical challenges aside, a universal online identity solution requires cooperation between private entities and government. SMA PROGRESS/Central Research Institute “WAVE” crypto labels system and blockchain technology for pharmaceutical industry is poised as the future of digital transactions, infusing trust, efficiency and transparency into drugs supply chains. Thus, proposed combination of crypto labels system and blockchains technologies, in sum, creates the only crypto labels system which may be called “secure”. The article proposes a perspective architecture based on the SMA PROGRESS/Central Research Institute “WAVE” Pharma Crypto Labels System architecture for the pharmaceutical industry.
References:
1. Patent of the RU No.72592 — Modern Identification Wireless Reader.//Mosienko S. A.
2. Patent of the RU No.37581 — Mobile telecom terminal.//Mosienko S. A.
3. Patent of the RU No.40123 — Mobile telecom terminal.//Mosienko S. A.
4. Patent of the RU No.41559 — Mobile telecom terminal.//Mosienko S. A.
5. Patent of the RU No.41219 — Mobile telecom terminal.//Mosienko S. A.
6. S. A. Mosienko, Crypto labels reader for future blockchain technology// “Young Scientist”. 2018. № 24, P. 39–42.
7. S. A. Mosienko, Crypto labels technologies integrating blockchain with automotive industry// “Young Scientist”. 2018. № 24, P. 36–39.
8. S. A. Mosienko, Breakthrough technologies: crypto labels and blockchain for pharmaceutical industry// “Young Scientist”. 2018. № 25, https://moluch.ru/archive/211/51740/
9. S. A. Mosienko, Blockchain technology for through-life asset management in the aviation/space/automobile industry//“Young Scientist”. 2018. № 24, P. 63–65.
10. Shekoufeh L. et al.// Parmazie,2012. Vol. N 10. P.817–821.
11. Security of Pharmaceutical. Comparing US and EU Standards (White Paper), 2016, Mettler-Toledo GmbH, PI-VI-WP-EN-GEN-PCE-092016.







